State of the art in Cassia grandis L. f. (cañandonga)
Estado del arte sobre Cassia grandis L. f. (cañandonga)
MSc. Ariadna Lafourcade Prada,I PhD. Jesús Rafael Rodríguez Amado,I PhD. Julio César Escalona Arranz,I MSc. Claudio Laurido FuenzalidaII
I University of Oriente. Santiago
de Cuba, Cuba.
II Faculty of Chemistry and
Biology. University of Santiago de Chile, Chile.
Introduction: The species Cassia grandis
L. f. (cañandonga) is recognized by the Cuban Health System and the population
for its antianemic properties, in spite of the unpleasant odor of its fruit.
Objectives: to perform a bibliographic update about the chemical, toxicological
and pharmacologic characteristics of the study species.
Methods: an extensive review was conducted in international databases such
as HighWire, DOAJ, EBSCO, Scielo, Scopus, Chemical Abstract, Medline, PudMed,
and Pharmaceutical Abstract, in addition to the national database CuMed from
the year 1900 until 2012.
Results: There are still not enough studies that certify its usefulness
and pharmaco-toxicological safety as antianemic, and few pharmaceutical formulations
have been developed. The fruit is the most studied organ of the species.
Conclusions: It is necessary to carry out new investigations to certify
its antianemic effect and develop new therapeutic alternatives to eliminate
the unpleasant odor of Cassia grandis L. f. fruit formulations.
Key words: Cassia grandis, antianemic, anemia, anthraquinones, alkaloids.
Introducción: la especie Cassia
grandis L. f. (cañandonga) es reconocida en el sistema de salud cubano
y su población por sus propiedades antianémicas, a pesar del desagradable
olor de sus frutos.
Objetivo: evaluar el estado del arte sobre aspectos químicos, toxicológicos
y farmacológicos de Cassia grandis L. f. (cañandonga) desde
1900 hasta 2012.
Métodos: se revisó en bases de datos internacionales
como HighWire, DOAJ, EBSCO, Scielo, Scopus, Chemical, Abstract, Medline,
PudMed, y Pharmaceutical Abstract, además de la base de datos nacional
CuMed desde 1900 hasta 2012.
Resultados: aún son insuficientes los estudios que avalan su utilidad
y seguridad farmacotoxicológica como antianémico, así como
pocas las formulaciones farmacéuticas desarrolladas. El fruto es el órgano
más estudiado de la especie.
Conclusiones: se necesita realizar nuevas investigaciones para avalar su
efecto antianémico y de otras alternativas terapéuticas que permitan
eliminar el olor desagradable de las preparaciones de los frutos de esta planta.
Palabras clave: Cassia grandis, antianémico, anemia, antraquinonas, alcaloides.
INTRODUCTION
Cassia grandis L. f. or cañandonga, as it is popularly known, has been traditionally used for the treatment of anemia.1 Native to the Americas (north, central, and south) and the Caribbean, it is a tree that measures up to 30 m in height and has thick branches. Despite being a plant recognized in the Formulario Nacional de Fito y Apifármacos (FNFA, 2010) of Cuba (FNFA),2 this official document does not declare enough studies that prove the antianemic and other ethnobotanical uses attributed to the plant.
This FNFA also presents a syrup as pharmaceutical
formulation, which is usually rejected by the Cuban population due to its unpleasant
odor, despite the benefits that the ethnobotanical use is attributed.
Considering these identified factors and with the aim of enriching the FNFA information, we conducted a review of the literature in relation to the usefulness, chemical composition, pharmacological and toxicological characteristics of this plant, as well as the preclinical, clinical and pharmaceutical formulations developed in the past.
METHODS
We conducted an exhaustive search for information in national database CuMed and international databases such as HighWire, DOAJ, EBSCO, Scielo, Scopus, Chemical Abstract, Medline, PudMed, and Pharmaceutical Abstract. The keywords entered in the "search options" were Cassia and Cassia grandis. Documents were considered when they described any type of pharmaceutical or ethnobotanical information. The data rank explored was from 1900 until 2012.
RESULTS
General characteristics
Cassia grandis L. f. is known in Cuba as cañandonga. Other common names are Coral shower, Apple blossom cassia, Pink shower, Liquorice tree and Horse cassia. It is a medium-sized tree, up to 20-30 m tall, found in abundance throughout tropical areas. Its leaves are compound and alternate. They are odorless and almost tasteless unlike the fruit, which has a strong smell and taste.
Ethnobotanical use
Decoction of the leaves, fruit and bark is used orally to treat anemias,3,4 nosebleeds, liver disease, urinary tract infections, hysteria, colds and coughs.5,6 Topically applied ointment from leaves is used to treat dermato-mucosal conditions (herpes, sores, tinea and vitiligo).7 From root extracts, a liquid antiseptic is obtained which is used in healing wounds.7 Also, the bark is used for healing.8 The leaves and fruit are attributed antianemic, antifungal, antiseptic, astringent, depurative, diuretic, stimulant, expectorant, febrifuge, galactogogue, laxative, mineralizing, pectoral, purgative, sedative and tonic effects. The juice of the leaves is used to combat ringworm. Decoction of the leaves is used as a laxative and for lumbago.1,9 Root preparations are attributed febrifuge, laxative and tonic effects. The bark of the trunk and large branches is believed to have antirheumatic properties and is used to treat skin conditions. In the Philippines, the leaves are used for fungal skin infections.8,9
Pharmacognostic studies
Only one paper was found related to the topic of leaves. Nevertheless, authors refer to the variability of drug constituents and physicochemical nature. They attribute those changes to various exogenous and endogenous factors, such as temperature, rainfall, light length, age of the plant, drying procedure, moisture and storage of samples. These observations should be taken into consideration to standardize the pharmaceutical formulation derived from this plant.10
Chemical composition
There is great variability in the composition of metabolites of the different organs of the plant, mainly due to differences in the conditions where they grow. Phytochemical screening of the leaves showed the presence of carbohydrates, alkaloids, sterols, anthraquinones, saponins, flavonoids, glycosides and tannins.11
Quantitative chemical characterization studies of the fruit dry powder showed the presence of triterpenes and steroids, essential oils, reducing sugars, amino acids, amines, saponins, polysaccharides and glycosides. Minerals like potassium, magnesium, cobalt, iron and nickel were also found.12 Anthraquinones are a type of metabolite widespread in the genus Cassia, and it has been reported in all plant organs. The leaves contain aloe-emodin, crisofanic acid, physcion and rhein.13 The fruit contains 1,3,4-trihydroxy-6,7,8-trimethoxy-2-methylanthraquinone; 3-O-ß-D-glucopyranoside.14 The stems contain emodin-9-anthrone;15 and the seeds contain chrysophanol; 1,2,4,8,-tetrahydroxy-6-methoxy-3-methylanthraquinone; 2-O-ß-D-glucopyranoside; 3-hydroxy-6,8-dimethoxy-2-methylanthraquinone; 3-O-ß-D-glucopyranoside and 1,3-dihydroxy-6,7,8-trimethoxy-2-methylanthraquinone-3-O- ß-D-glucopyranoside.16
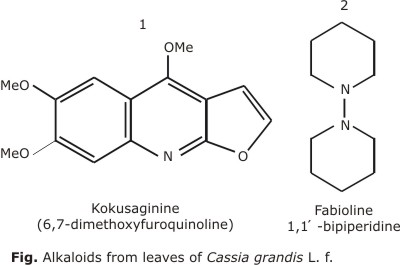
Another group of metabolites reported in various organs of the plant are the alkaloids. Leaves are reported to contain alkaloids such as kokusaginine (6, 7-dimetoxyfuroquinoline) and 1, 1-bipiperidine.17 These compounds are also reported in the fruit.18 Figure shows the chemical structure of both alkaloids.
From the leaves of Cassia grandis L., several C6-C3 compounds were isolated, such as trans-3-methoxy-4,5-methylene-dioxycinnamaldehyde; 2,4-dihydroxy benzaldehyde; 3,4,5-trimethoxybenzaldehyde; 2,4,6-trimethoxybenzaldehyde.18 The leaves also contain barakol, kaempferol, leucoantocianidines and saponins.19,20 The foliage contains polyphenols (5.61 %), tannins (3.59 %), tannins precipitable proteins (3.64 %), and hydrolysable tannins (0.28 %).21,22
The fruit is reported to contain cinnamic acid
and sugars.23 The presence of saponins, phenols, tannins and amino
acids is also reported.24 Other compounds isolated from the fruit
are centaureidin, cathechin, myristicin, 2,4-dihydroxibenzaldehyde; 3,4,5-trimethoxybenzaldehyde;
2,4,6-trimethoxy benzaldehyde and ß-sitosterol.18 Studies of
the volatile fractions of this organ, in species growing in
Cuba show that this is a large plant producing oils with a total of 108 compounds,
and report a yield of 30.47 mg/kg. A major component is linalool, with 31.5
% of the volatile components.25
The seeds contain flavonoids and polysaccharides,8,26
especially a pure galactomannan with a mannose:galactose ratio of 3:15.27 The
endosperm of the seed contains 50 % of a gum, which was used
as binder in the tablet manufacturing process.28 The stems contain mainly three
compounds: palmitic acid, ß-sitosterol and emodin-9-anthrona.19,29
The plant exudate has acidic properties and a high content of glycine, xylose
and galacturonic acid.30
DISCUSSION
Antianemic activity
Despite being the most commonly reported ethnobotanical use of the plant, the only reference found in the review was to Tillán et al.31 These researchers evaluated the antianemic effect of fruit dry powder in an anemia experimental model of iron deficiency in rats. They compared three groups: one without any supplement, one supplementing the diet with 15 mg/kg of iron, and one receiving the same amount of iron plus 750 mg/kg of body weight, of the dry powder of the fruit of Cassia grandis L. f. Average concentrations of hemoglobin in the group supplemented with iron and Cassia grandis L. f. were higher than in the other groups. A significant increase in plasma iron values was also observed. The results corroborated the popular and traditional use of Cassia grandis L. f. fruit in anemic states to improve the utilization of iron in hemoglobin production.32
Antifungal activity
The ethanolic extract of leaves and bark showed in vitro antifungal activity against Epidermophyton floccosum, Microsporum gypseum and Trichophyton rubrum in pure cultures, with a minimum inhibitory concentration of 50 µg/mL.32
Antidiabetic activity
Antidiabetic activity was evaluated and the glucose tolerance test of aqueous and ethanolic extracts of the stem was conducted. In both cases, Sprague Dawley rats were used. In the test of tolerance to glucose, both extracts significantly reduced blood glucose to normal levels. In the test of diabetes induced by alloxan, maximal reduction of glucose occurred at three hours with a dose of 150 mg/kg. The essay demonstrated a strong antidiabetic effect of extracts of this plant.33
Antiinflammatory and analgesic activity
Antiinflammatory and antinociceptive activity of methanolic extracts of the leaves of the plant was evaluated. Analgesic activity was tested by evaluating the central and peripheral pharmacological action. The Eddy´s hot plate method and the contortions methods induced by acetic acid were used. Anti-inflammatory activity was evaluated using a digital plethysmometer. Doses of 100 mg/kg, p.o. were used. In all cases, significant analgesic activity with a good antiinflammatory profile was observed.34
Antioxidant activity
Antioxidant activity in vitro was reported for various extracts from leaves of this plant. The free radical scavenging activity like DPPH, nitric oxide and hydroxyl radical was evaluated. Butylated hydroxytoluene (BHT) was used as reference standard. In all cases, the methanolic extract significantly inhibited antioxidant activity. In chloroform and ether extracts this activity was not observed.10
Oral acute toxicity
Acute toxicity of three oral dosage forms prepared from the dried powder of the fruit was reported by Lagarto & Guerra. The assay was performed to a limit test dose of 2 000 mg/kg body weight, in Wistar rats of both sexes. In the study, the 3 formulations classified as non-toxic, since no signs of toxicity were observed at the dose level and experimental conditions.35
Genotoxic study
A toxicogenetic study of fluid extract from leaves of Cassia grandis L. f. was performed. Two systems are short-term tests: model A. niger D-30, which detects primary DNA damage assay, and micronucleus induction in mouse bone marrow, which evaluates clastogenic and aneugenic damage. No genotoxic activity was observed in either test battery.24
Reported dosage forms
The Cuban Formulario Nacional de Fito y Apifármacos (FNFA) presents two formulations: the fluid extract and 10 % syrup.2 Another source reported the toxicological evaluation of three dosage forms: Ferroscassia dry drug and Powder for infusion (just stating that it is prepared from the fruit, apparently fresh) and "Instaferros", prepared from the dried fruit, with added sugar and flavoring. However, there was no report on the manner in which these preparations are made.35 No other pharmaceutical forms are reported for extracts of this plant.
Other studies
It has been reported that infusion of the leaves has no diuretic activity in rats.35 The seeds of Cassia grandis L. f. inhibit the mutagenic activity of 1,1-diphenyl-2-picrilhydrazil, with IC50= 1 108 µg/mL.36
Despite the potential this plant has shown, especially the fruit, and considering it is a drug included in the Cuban FNFA, insufficient studies have been performed. Further research is needed to confirm its ethnobotanical uses. Similarly, new pharmaceutical forms are needed to improve patient acceptability of this medicinal plant.
REFERENCES
1. Roig JT. Plantas medicinales aromáticas o venenosas de Cuba. La Habana: Editorial Científico-Técnica; 1988. p. 263-4.
2. FNFA. Formulario Nacional de Fito y Apifármacos. Ministerio de Salud Pública de Cuba. la Habana: Dirección Nacional de Farmacias; 2010. p. 50-2.
3. Morton JF. Some folk-medicine plants of Central American Markets. Quart J Crude Drug Res. 1977;15(4):165-92.
4. Bonzani RM. Medicinal use of plants by the peasant community of San Jacinto, northern Colombia. Etnobiol. 1999;21(2):203-18.
5. Standlcy PC, Stcycrmark JA. Flora of Guatemala. Fieldiana: Botany. 1946;24(5):116.
6. House P, Lagos-Wittc S. Manual de 50 plantas medicinales de Honduras. Tegucigalpa, Honduras: CONSH/CIIR/UNAH; 1989. p. 48.
7. Nelson CH. Plantas comunes de Honduras. Tegucigalpa, Honduras: Editorial Universitaria; 1986. p. 264.
8. Cáceres A. Plantas de uso medicinal en Guatemala. San Carlos de Guatemala. Guatemala: Editorial Universitaria; 1996. p. 115-6.
9. Toruan-Purba AV. Cassia Linn. En: de Papua LS, Bunyapraphatsara N, Lemmens RHMJ, editors. Plant Resources of South-East Asia. No. 12(1): Medicinal and poisonous plants. Leiden, Holanda: Backhuys Publisher; 1999. p. 181-5.
10. Meena MK, Gaur K, Kori ML, Sharma CS, Nema RK, Jain AK, et al. In-vitro antioxidant properties of leaves of Cassia grandis Linn. Asian J Pharm Clin Res. 2009;2(1):46-9.
11. Meena MK, Pal SC, Jain AK, Jain CP, Ashawat MS, Gaur K, et al. Comparative study on physicochemical variation for different samples of Cassia grandis Linn. Leaves. Afr J Plant Sci. 2010;4(7):261-7.
12. Águila Y. Caracterización de una materia prima con propiedades antianémicas, a partir de un producto natural [Trabajo de Diploma]. La Habana: Instituto de Farmacia y Alimentos, Universidad de La Habana; 1999.
13. Ambasta BK, Prassad G, Sinha KS, Verma RP. An anthraquinone derivative from Cassia grandis Linn. Indian J Chem. 1996;35(9):990-1.
14. Vermaa RP, Sinhaa KS. An Anthraquinone from Cassia grandis Linn. Nat Prod Lett. 1994;5(2):105-10.
15. Meena R, Kalidhar SB. Chemical examination of the stems of Cassia grandis L. Indian J Pham Sci. 1998;60(1):59-69.
16. Siddiquia IR, Singha M, Gupta D, Singha J. Anthraquinone-O-ß-D-Glucosides from Cassia grandis. Nat Prod Lett. 1993;2(2):83-90.
17. Valencia E, Madivaveitia A, Bermejo J, González AG, Gupta MP. Alcaloids from Cassia grandis. Fitoterapia. 1995;16(1):476-7.
18. González AG, Bermejo J, Valencia E. A new C6-C3 compound from Cassia grandis. Planta Med. 1996;62(2):176-7.
19. Glasby JS. Dictionary of plants containing secondary metabolites. Londres. UK: Taylor and Francis; 1991. p. 67.
20. Dave H, Ledwani L. A review on anthraquinones isolated from Cassia spp. and their applications. Indian J Nat Prod Res. 2012;3(3):291-319.
21. García DE, Medina MG, Humbría J, Domínguez C, Baldizán A, Cova L, et al. Composición proximal, niveles de metabolitos secundarios y valor nutritivo del follaje de algunos árboles forrajeros tropicales. Arch Zootech. 2006;55(212):373-84.
22. García DE, Medina MG. Composición química, metabolitos secundarios, valor nutritivo y aceptabilidad relativa de diez árboles forrajeros. Zootecnia Tropical. 2006;24(3):233-50.
23. Correa QJE, Bernal MHY. Especies vegetales promisorias de los países del convenio Andrés Bello. Bogotá, Colombia: convenio Andrés Bello; 1990. p. 485.
24. Vizoso PA, Ramos RA, García LA, Piloto FJ, Pavón GV. Estudio genotóxico in vitro e in vivo del extracto fluido de Cassia grandis L. y el gel de Aloe vera L. Rev Cubana Plant Med. 2000;5(3):91-6.[Citada 28 Ene 2013]. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962000000300005&lng=es
25. Pino JA. Volatile Compounds of Cassia grandis L. f. fruit from Cuba. J Essent Oil Res. 2010;22(6):599-601.
26. Gritsanapan W, Tantisewie B, Jirawongse V. Chemical constituents of Cassia timorensis and Cassia grandis. J Sci Soc Thailand. 1984;10(3):189-90.
27. Joshi H, Kapoor VP. Cassia grandis L. f. seed galactomannan: structural and crystallographical studies. Carbohyd Res. 2003;338(19):1907-12.
28. Kharkwal H. Binding agents from Cassia species. Int J Pharm Phytopharmacol Res. 2012;2(2):83-6.
29. Meena R, Kalidhar SB. Chemical examination of the stems of Cassia grandis L. Indian J Pharm Sci. 1998;60(1):59-60.
30. Anderson DMW, Weiping W, Lewis GP. The composition and properties of eight gum exudates (Leguminosae) of American Origin. Biochem Syst Ecol. 1990;18(1):39-42.
31. Tillán CJ, Rodríguez CJ, Gómez MJM, Pardo RZ, Agüero FS. Actividad antianémica de la Cassia grandis L. Rev Cubana Farm. 2004;38(3):130-3. [Citada 22 Ene 2013]. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0034-75152004000300009&lng=es
32. Cáceres A, Lopez B, Juárez X, del Aguila J, García S. Evaluation of antifungal activity of seven American plants. J Ethnopharmacol. 1993;40(3):207-13.
33. Lodha SR, Joshi SV, Vyas BA, Upadhye MC, Kirve MS, Salunke SS, et al. Assessment of the antidiabetic potential of Cassia grandis using an in vivo model. J Adv Pharm Tech Res. 2010;1(3):330-3.
34. Linn AKJ, Jain CP, Gaur K, Jain A, Nema RK. Evaluation of antinociceptive and anti-inflammatory activity of leaves of Cassia grandis. IJPCR. 2010;2(3):106-8.
35. Lagarto PA, Guerra SMI. Toxicidad aguda oral de tres formas farmacéuticas a partir de Cassia grandis L. Rev Cubana Plant Med. 2000;5(2):68-70. [Citada 2 Feb 2013]. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962000000200009&lng=es
36. Ramos A, Visozo A, Piloto J, García A, Rodríguez CA, Rivero R. Screening of antimutagenicity via antioxidant activity in Cuban medicinal plants. J Ethnopharmacol. 2003;87(2-3):241-6.
Recibido: 13 de febrero de 2013.
Aprobado: 17 de octubre de 2013.
Ariadna Lafourcade Prada. Universidad de Oriente. Patricio Lumumba S/N. Santiago de Cuba. Cuba. Teléf.: 632263. E-mail: ariadna@cnt.uo.edu.cu