ARTÍCULO ORIGINAL
Effect of an extract from Plumbago scandens L. (malacara) on Chrysomya putoria post-embryonic development
Efecto de un extracto de Plumbago scandens L. (malacara) sobre el desarrollo post embrionario de Chrysomya putoria
MSc. Marcio Borges Pinto Lopes,I,II Dra. C. Maria Raquel Figueiredo,III Dra. C. Ana Luiza Rangel Bérenger,III Dra. C. Selma Paiva,III,IV Dr. C. Julio César Escalona Arranz,V Dra. C. Margareth Maria de Carvalho QueirozI
I
Laboratório de Transmissores de Leishmanioses - Setor de Entomologia
Médica e Forense /IOC, Fundação Oswaldo Cruz, FIOCRUZ, Rio
de Janeiro, RJ, Brasil.
II Programa de Pós-graduação em Biologia Animal
- Universidade Rural do Rio de Janeiro/UFRRJ, Rio de Janeiro, RJ, Brasil.
III Laboratório de Química de Produtos Naturais - Instituto
de Tecnologia em Fármacos, Fundação Oswaldo Cruz, FIOCRUZ,
Rio de Janeiro, RJ, Brasil. IV
Setor de Botânica, Departamento de Biologia Geral, Instituto de
Biologia, Universidade Federal Fluminense, Niterói, RJ, Brasil.
V Departamento de Farmacia. Universidad de Oriente, Santiago
de Cuba, Cuba.
ABSTRACT
Introduction:
excessive use of chemical insecticides for pest control has become a dangerous
practice, for these products may affect both human beings and animals. This
is the reason why the development of biopesticides has gained great importance.
Plumbago scandens L. (malacara) is a medicinal plant producing
large amounts of plumbagin, a compound with high larvicidal activity against
mosquitoes.
Objective:
evaluate the toxicity / selectivity of a P. scandens extract (PSE)
on the post-embryonic development of the Chrysomya putoria fly.
Methods:
a crude extract was prepared from dry roots of the species by Soxhlet extraction
in n-hexane. Chemical identification of plumbagin in the extract was based
on GC/MS. Three concentrations (25 %, 50 % and 75 %) were evaluated, monitoring
the viability and duration of each stage in the life cycle of the fly (larval,
pupal and development until adulthood). An evaluation was also conducted of
the effects on the weight of mature larvae and the sex ratio.
Results:
none of the PSE concentrations evaluated differs from the control group at
the larval or pre-adulthood development stages, but they do differ slightly
at the pupal stage in the 25 % and 50 % groups. There were no variations in
the sex ratio, but differences were found in larval weight, which was lower
in the 50 % and 75 % groups.
Conclusion:
the PSE does not have a relevant effect on any of the stages in the lifecycle
of the fly. Therefore, it is not declared to be toxic on the biological model
used, thus meeting one of the most important attributes of a biopesticide:
its biological selectivity.
Key words: Chrysomya putoria, Plumbago scandens, flies, biopesticides, plumbagin.
RESUMEN
Introducción:
el excesivo uso de insecticidas químicos para el control de insectos
se ha convertido en una práctica peligrosa, pues esos productos pueden
afectar al hombre y otros animales, es por ello que el desarrollo de biopesticidas
ha cobrado gran importancia. Plumbago scandens L. (malacara) es una
planta medicinal que produce grandes cantidades de plumbagina, un compuesto
con elevada actividad larvicida en mosquitos.
Objetivo:
evaluar la toxicidad/selectividad de un extracto de P. scandens (PSE)
sobre el desarrollo post embrionario de la mosca Chrysomya putoria.
Métodos:
se preparó un extracto crudo por extracción con soxhlet en n-hexano
a partir raíces secas de la especie. La identificación química
de la plumbagina en el extracto fue realizada por GC/MS. Tres concentraciones
diferentes (25, 50 and 75 %) fueron evaluadas, monitoreando la viabilidad
y la duración de cada etapa del ciclo de vida de la mosca (larval, pupal
y desarrollo hasta la adultez). También se evaluaron los efectos sobre
el peso de las larvas maduras y la razón sexual.
Resultados:
ninguna de las concentraciones de PSE evaluadas difieren del grupo control
en las etapas larval y de desarrollo hasta la adultez, pero si lo hacen ligeramente
en el período pupal en los grupos de 25 y 50 %. Tampoco varió la
razón sexual, pero sí se observaron diferencias con respecto al
peso de las larvas, los cuales fueron inferiores en los grupos de 50 y 75
%.
Conclusión:
el PSE no muestra un efecto importante en ninguna de las etapas del ciclo
de vida de la mosca, es por ello que no se declara tóxico ante el modelo
biológico empleado, cumpliendo con uno de los atributos más importantes
de un biopesticida: la selectividad biológica.
Palabras clave: Chrysomya putoria, Plumbago scandens, moscas, biopesticidas, plumbagina
INTRODUCTION
An excessive use of chemical insecticides for insect control have been raise nowadays, becoming especially dangerous since these products can affect man and others animals, pollute the air, water and even enter the food chain. Actually it is recognized the increase of the insect resistance degree to those chemical products, generating a more abusive (quantities) use of those substances. Other less mentioned negative side effects also appear: the poor biological selectivity, creating an additional problem to the biodiversity, due to the extermination of various kinds of insects. Thus, any alternative form of insect control has become important.
Plant and their natural enemies (insects, bacteria or viruses) have undergone a co-evolution process in which a new plant resistance character that reduces enemy attack is developed. At the same time plants also need the contribution of insects to realize functions related to plant develop, such as pollination.1 By this way, plant metabolites are synthesized for both attraction and repellent/deterrent functions. This natural selectivity should been useful to develop some plant biopesticides.
Biopesticides provide an alternative because they have low impact on the environmental, low toxicity to humans, low costs as well as other advantages.2 Furthermore, unlike conventional commercial insecticides that usually are based on single active ingredient, plant-derived insecticides comprise botanical blends of secondary metabolites which act concertedly on both behavioral and physiological processes of the target pests. Thus, the chances of pests developing resistance to such substances are meagre.3
Plumbago species are reported in the literature for its biological activities such as: antiparasitic,4 insect antifeedant,5 antibacterial,6 antifungal, anti-inflammatory7 and anticoagulant. Those activities are usually assigned due to the presence of special chemical compounds, such as naphthoquinones, substances which are also effective against insects.8 Plumbagin is a naphthoquinone well distributed among Plumbago species, specially found in their roots.9
According to Kishore, the compound plumbagin compared with other natural compounds with larvicidal activity, is very toxic against mosquito larvae of the species Anopheles gambiae Giles , 1926 and Aedes aegypti Linnaeus, 1762 and it would be a potential source of natural substances and be used as biopesticide.10
Plumbago scandens L. (Plumbaginaceae) is a subshrub with white flowers quite widespread in the Tropical Continental America and the Antilles. Cuba and Brazil are two of those countries in which this plant can be found. It is a native species found in typical vegetation characterized by high luminous intensity, sandy soil and water restriction. Even when no many scientific papers have been published about this plant, it is known that the chemical profile of the genus is marked by the presence of naphthoquinones, flavonoids and terpenoids,11 in which plumbagin is the major compound.12
Chrysomya putoria (Wiedemann, 1818) (Diptera: Calliphoridae) this species of blowfly has a considerable sanitary importance, because it can be a mechanical vector of pathogens;1 and it was also record as myiasis producer in man and animal.14 Is considered as one of the first invertebrates to arrive at and begin feeding on putrefied organic matter, playing a significant role in the decomposition and reprocessed of the organic matter and giving and additional forensic important. They can survive in different habitats, since they are ecologically diverse. The larvae are capable of developing on various substrates, including feces, garbage and corpses.15
Therefore, studies that they evaluate the toxicity or selectivity of plant extracts in which plumbagin is present on the post embryonic development of C. putoria, it looks as a good effort to find promissory biopesticides.
METHODS
Plant Material
Roots from healthy plants of Plumbago scandens L. were collected at Fundação Oswaldo Cruz campus at morning time, Rio de Janeiro State, Brazil. A voucher of this plant was deposited at Rio de Janeiro Botanical Garden Herbarium (RB) under the number 340.340, being identified by R. J. Paixão.
Plant extraction
We followed the same procedure that previous papers published by members of the team.16 Fresh roots of P. scandens were oven dried at 40 °C and powdered (450g). After, they were exhaustively extracted with chloroform for 10 h in a Soxhlet apparatus. The solvent was eliminated under reduced pressure in a BüchiR114 apparatus at 40 °C, to reach the dry crude extract.
Chemical identification of plumbagin on the extract
GC/MS was performed on a Hewlett-Packard gas chromatograph model 6890N equipped with a mass selective detector, model 5973, and an automatic injector model 5683, as well as a capilar column HP-5MS (5 % phenyl, 95 % methyl syloxan), 30 m × 0.25 mm × 0.25 μm. Data acquisition was performed by HP Chemistation Data Acquisition Software. The following conditions were used: helium as carrier gas, mass detector, detector temperature = 280 °C, injector temperature = 270 °C, flow rate 1.0 mL/min, split of 1:20, injection volume = 1.0 μL, initial temperature = 120 °C and oven program from 5 °C/min to 290 °C followed by an isotherm period of 20 min. A previously isolated and characterized plumbagin was used as internal standard to confirm the presence of this compound on the extract.17
Biological experiment
Fly collection and maintenance
The blowflies C. putoria were collected on the campus of Fundação Oswaldo Cruz, Rio de Janeiro, and were reared and maintained at the Laboratório de Transmissores de Leishmanioses - Setor de Entomologia Médica e Forense of the same Institution following the methodology used in previous works according to previous experiences of our team.18 The cultures used here were kept in cages at room temperature with water and sugar ad libitum. Protein in the form of rotting groundwood bovine meat was given for maturation of the ovarioles and to stimulate oviposition. The second generation was reared following the same methodology and newly hatched larvae were used in the experiments.
Laboratory bioassays
The dry crude extract of P. scandens was dissolved in hot distilled water until reach concentrations equivalent to 0.25, 0.5 and 0.75 milligram of dried extract per milliliter. Those experimental solutions were marked as 25, 50 and 75 %. The plat extract at those concentration were applied topically in groups of 50 newly hatched larvae each (1 μL/ newly hatched larvae). Three repetitions were performed for each bioassay, plus one control group consistent in distilled water, totaling 600 larvae. Newly-hatched larvae were grouped in a Petri dish with filter paper moistened with distilled water with a small piece of bovine meat in the center to keep the larvae grouped together. In the experimental and control groups, the paper with the larvae was transferred and placed onto putrefied bovine meat (50 g), with a proportion of 1g of meat for each larva, to guarantee enough food for maximum development. These recipients (100 mL) were then placed into larger recipients (500 mL) containing vermiculite as a substrate for pupation and then covered with a nylon cloth held down with rubber bands. After reaching maturity, the larvae spontaneously abandoned the diet, falling onto the vermiculite. These larvae were individually weighed in analytical balance after the abandon of the diet and transferred to glass tubes containing vermiculite to one-fourth of their volume and closed with cotton plugs. The experiments were maintained in acclimatized chambers set at 27±1 °C, 70±10 % RH, 12:12 light/dark cycle. Daily observations were made until the emergence of the adults, with subsequent sexing and morphologic analysis if there a changes.19
The viability and duration of each period (larval, pupal and newly-hatched larvae to adult) were analyzed. Other variables considered were the weight of mature larvae and the sex ratio of the adults.
Data analysis
The results were analyzed with the help of different statistical test supported in the professional packaged STATGRAPHICS Centurion XV, Version 15.02.05. StatPoint, Inc. 2006. Viability differences were calculated by the W test of Mann-Whitney (Wilcoxon), stages duration and larval weight by the Tukey–Kramer test, both at the 0.05 (%) significance level; and the sex ratio was tested by chi-square.
RESULTS
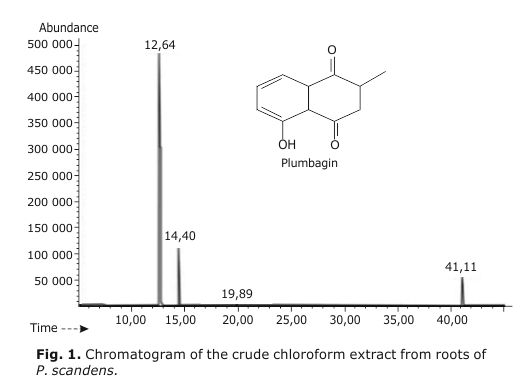
As result of the extraction procedure, 7.1 g of dry crude extract was obtained, meaning a total yield of 1.58 %. A small portion of this amount, was dissolved in ethyl acetate and injected into the before mentioned gas chromatograph coupled with a mass spectrometer. Under those conditions, four peaks appear, in which the main one on behalf of plumbagin with 71.86 % and retention time of 12.64 min (figure 1). In addition to plumbagin that represent the 71.86 % of the isolated compounds, other three compounds are also detected in the P. scandens extract (PSE) representing the 17.78, 8.89 and 1.46 % and corresponding to other quinone types metabolites which has been already described in previous papers.16
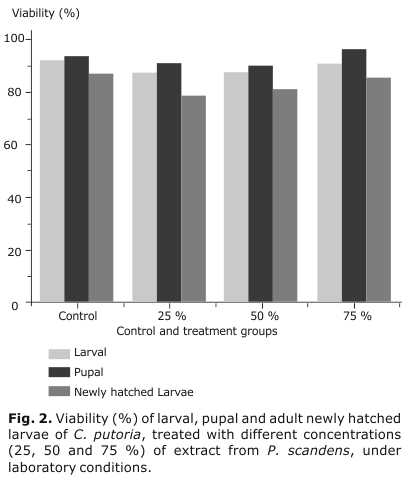
The viability of the control group for all periods of development (larvae, pupae and newly hatched larvae to adult) was 92, 94, and 87 %, having a similar behavior that the three experimental groups according to the statistical test accomplished (Mann-Whitney, α=0.05) and as can be appreciated in figure 2.
Aspects regarded to the days needed to reach each one of the fly’s development stages are presented in table 1, meanwhile those related to the larval weight as well as the sexual ratio are displayed on table 2.
Regarding to the viability, the high values observed for this variable in the control group can interpreted as good experiment conditions to the developed of flies. In all cases the viability of the control group was similar to the three experimental groups, with the only exception at the 25 and 50 % concentrations, in which the newly hatched larvae period gives 79 and 81 % of viability. Nevertheless, we cannot consider those values as relevant or important as insecticide activity due to the “in vivo” conditions of the experiment.
As it can be appreciate in table 1, experimental groups do not statistically differ to the control group in the times needed to reach the Larval and newly hatched larvae to adult stages. The only stage in which the P. scandens extract (PSE) has evidence to get some influence is in pupal stage and only at the 25 and 50 % experimental groups. In those cases, the time to pass to the other stage was smaller without exhibit visual consequences. In addition, is notorious that the higher concentration (75 %) attains the normal behavior. Also, the influence of all experimental groups in this pupal stage did not affect the succeeding flies develop stage (newly hatched larvae to adult), minimizing the possible toxicity of the PSE on the C. putoria cycle of life.
Table 2 contains aspects regarding to the larval weight as well as the sexual ratio are. In one hand, the sexual ratio is conserved almost in the same proportion for all experimental groups, meaning a non demonstrable influence of PSE on this variable. On the other hand, larval weight variable is evidently different in 50 and 75 % groups related to the control group. It is also significative the difference in the range of weight, including even the first experimental group (25 %) in which no statistical differences were found.
DISCUSSION
In general, the plant extracts considered as good biopesticides do not affect all the flies’ stages, being specifically toxic in one of the variables monitored, but as a cycle; one break is good enough to disrupt the develop of the insect.20,21
PSE do not exhibit an important effect in no one of fly stages, that’s why attending to other studies in which plant extracts are tested as potential biopesticides, we cannot declare PSE as toxic to the biological model employed. This behavior contrast with the observed again mosquito larvae; in which plumbagin prove to be a good larvicidal.10
The results obtained in this experiment cannot be considered as negative, because one of the most important values of a good biopesticide is to be selective to the species that must be controlled, and do not affect in the development of other species that can be important for the biodiversity of the area. This fact acquires more relevance, when the insecticidal activity block the reproduction ratios, more than the insect's elimination; because the substance under investigation act as regulator of population without affect the biological role of the specie and without having a notorious impact in the nutritious chain.
In general, the results of this study show that P. scandens extract (PSE) do not exhibit a high toxicity on the post embryonic development of C. putoria, demonstrating that PSE, and particularly plumbagin can be selective to diverse insect types, rising as a potential biopesticide.
ACKNOWLEDGMENTS
The authors are grateful for the financial support received from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Office for the Advancement of Higher Education) and CAPES-MES program (Brazil-Cuba).
REFERENCES
1. Mark D. Rausher. Co-evolution and plant resistance to natural enemies. Nature. 2001;411(June):857-864.
2. Rajkumar S, Jebanesan A. Larvicidal and oviposition activity of Cassia obtusifolia Linn (Family: Leguminosae) leaf extract against malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res. 2009;104:337-340.
3. Rawani A, Haldar KM, Ghosh A, Chandra G. Larvicidal activities of three plants against filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res. 2009;105:1411-1417.
4. Salomão K, Santana N, Molina MT, Castro S, Menna-Barreto RFS. Trypanosoma cruzi mitochondrial swelling and membrane potential collapse as primary evidence of the mode of action of naphthoquinone analogues. BMC Microbiology 2013, DOI: 10.1186/1471-2180-13-196.
5. Villavicencio MA, Perez-Escandon BE. Plumbagin activity (from Plumbago pulchella Boiss. Plumbaginaceae) as a feeding deterrent for three species of Orthoptera. Folia Entomol Mex. 1992;86:191-198.
6. Dey D, Ray R, Hazra B. Antitubercular and Antibacterial Activity of Quinonoid Natural Products against Multi-Drug Resistant Clinical Isolates. Phytother Res. 2013. Dec 6. doi: 10.1002/ptr.5090.
7. Checker R, Patwardhan RS, Sharma D, Menon J, Thoh M, Sandur SK, et al. Plumbagin, a Vitamin K3 analogue, abrogates Lipopolysaccharide-Induced Oxidative Stress, Inflammation and Endotoxic Shock via NF-kB Suppression. Inflammation. 2014;37(2). DOI: 10.1007/s10753-013-9768-y.
8. Pradeepa V, Narayanan SS, Kirubakaran SA, Nathan SS. Antimalarial efficacy of dynamic compound of plumbagin chemical constituent from Plumbago zeylanica Linn (Plumbaginaceae) against the malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res. 2014, 113: 3105-3109. DOI 10.1007/s00436-014-4015-5.
9. Gala AM, Ramam V, Avula B, Wang YH, Rumalla ChS, Weerasooriya AD, et al. Comparative study of three Plumbago L. species (Plumbaginaceae) by microscopy, UPLC-UV and HPTLC. J Nat Med. 2012. DOI 10.1007/s11418-012-0717-0.
10. Kishore N, Mishra BB, Tiware VK, Tripathi V. An account of phytochemicals from Plumbago zeylanica (Family: Plumbaginaceae): A natural gift to human being. Chronicles of Young Scientists. 2011;3(3):178-198.
11. Paiva SR, Figueiredo MR, Lima LA, Kaplan MAC. Chemical composition fluctuations in roots of Plumbago scandens L. in relation to floral development. An Acad Bras Ciênc. 2011;83(4):1-6.
12. Paiva SR, Lima LA, Figueiredo MR, Kaplan AC. Plumbagin quantification in roots of Plumbago scadens L. obtained by different extraction techniques. An Acad Bras Ciênc. 2004;76:499-504.
13. Carriço C, Pinto ZT, Dutok CM, Caetano RL, Pessanha RR, Chil-Nuñez I, et al. Biological activity of Pouteria sapota leaf extract on post-embryonic development of blowfly Chrysomya putoria (Wiedemann, 1818) (Calliphoridae). Rev Bras Farmacogn. 2014,24(3):304-308.
14. Corrêa EC, Koller WW, Barros ATM. Chrysomya (Diptera: Calliphoridae) relative abundance and species seasonality in the Pantanal, State of Mato Grosso do Sul, Brazil. Rev Bras Parasitol Vet. 2010;19(2):85-88.
15. Queiroz MMC, Mello RP, Freire MS. The effect of different proportions the Chrysomya albiceps (Wiedemann 1819). (Diptera, Calliphoridae) biotic potential and longevity under laboratory conditions. Mem Inst Osw Cruz. 1996;91:243-247.
16. Paiva SR, Fontoura LA, Figueiredo MR. Perfil cromatográfico de duas espécies de Plumbaginaceae: Plumbago scandens L. e Plumbago auriculata Lam. Quim Nova. 2002;25(5): 717-721.
17. Paiva SR, Lima LA, Figueiredo MR, Kaplan MAC. Chemical composition fluctuations in roots of Plumbago scandens L. in relation to floral development. An Acad Bras Ciênc. 2011;83(4):1165-1170.
18. Mendonça PM, Queiroz MMC, d'Almeida JM. Rearing Chrysomya megacephala on artificial diets composed of varying concentrations of albumin. Braz Arch Biol Technol. 2009;52(2):421-426.
19. Ferraz ACP, Dallavecchia DL, Silva DC, Carvalho RP, Silva Filho RG, Aguiar-Coelho VM. Alternative diets for Chrysomya putoria, an Old World screwworm fly. J Insect Sci. 2011;43(12):1-11
20. Cabral MMO, Mendonça PM, Gomes CMS, Barbosa-Filho JM, Dias CS, Soares MJ, et al. Biological Activity of Yangambin on the Postembryonic Development of Chrysomya megacephala (Diptera: Calliphoridae) J Med Entomology. 2007;44(2):249-255.
21. Mendonça PM, Lima MA, Albuquerque LRM, Carvalho MG, Queiroz MMC. Effects of latex from “Amapazeiro” Parahancornia amapa (Apocynaceae) on blowfly Chrysomya megacephala (Diptera: Calliphoridae) post-embryonic development. Vet Parasitol. 2011;178:379-382.
Recibido: 2 de
abril de 2014.
Aprobado:
10 de octubre de 2014.
Dr. C. J.C. Escalona Arranz Tel.: +53 (22) 641411, +53 52371348 Correo electrónico: jcea@cnt.uo.edu.cu