ORIGINAL ARTICLE
Achyrocline satureioides (Lam.) DC. (marcela) reduces brain damage in permanent focal ischemia in rats
Achyrocline satureioides (Lam.) DC. (marcela) reduce el daño cerebral en la isquemia focal permanente en ratas
PhD. Felicia Rivera Megret,I,II,III BSc. Dario Tejera Correa,I,II PhD. Juan Andrés Abin Carriquiri,I PhD. Giselle Prunell dos Santos,I MSc. Marcela Martínez Busi,I Prof. Federico Dajas MéndezI,II
I Biological Research. Institute Clemente
Estable. Montevideo. Uruguay.
II Associated Unit of Neurochemistry.
Faculty of Science. University of the Republic, Montevideo. Uruguay.
III Faculty of Medicine CLAEH.
Uruguay.
Introduction: Achyrocline
satureioides is a plant which has been widely used in popular medicine
and experimental studies confirm its antioxidant and anti-inflammatory effects,
attributable to the presence of flavonoids, mainly quercetin.
Objectives: to evaluate the neuroprotective effects of a chronic oral pre-administration
to rats with an Achyrocline satureioides decoction (2 %).
Methods: for decoction, dried flowers of Achyrocline satureioides
were used. The consumption of food and AS decoction/water of the rats was evaluated
daily and weight gain weekly; quercetin content in the decoction and in
the plasma of the rats was evaluated by high performance liquid chromatography.
The cerebral damage was assessed with a tetrazolium salt (TTC) and a behavioral
test was performed previously. Nissl staining and Fluoro-Jade histochemistry
were used.
Results: the pre-treatment with Achyrocline satureioides in all groups
reverted the functional deficit and, during 21 days, the infarction volume also
decreased significantly. Nissl staining showed a higher percentage of preserved
neuronal population and the Fluoro-Jade showed a decreased of the neurons in
degeneration.
Conclusions: the quercetin levels in the decoction and plasma of rats could
explain the preventive benefits of Achyrocline satureioides due to the
antioxidant and anti-inflammatory properties described for this flavonoid.
Key words: Achyroclines satureioides, quercetin, permanent focal ischemia.
Introducción: Achyrocline satureioides
es una planta que ha sido ampliamente utilizada en la medicina popular y los
estudios experimentales confirman sus efectos antioxidantes y antiinflamatorios,
atribuibles a la presencia de flavonoides, principalmente quercetina.
Objetivos: evaluar los efectos neuroprotectores de la pre-administración
oral crónica a ratas con una decocción de Achyrocline satureioides
2 %.
Métodos: para la decocción se utilizaron flores secas de Achyrocline
satureioides. Se cuantificaron, diariamente, el consumo de alimentos, la
decocción y el agua; y cada semana, la ganancia de peso. El contenido
de quercetina en la decocción y en el plasma de las ratas se evaluó
utilizando la técnica de cromatografía líquida de alta
resolución. El daño cerebral se cuantificó con una sal
de tetrazolio y antes se realizó una prueba de comportamiento. Se utilizaron
la tinción de Nissl y el fluoro Jade.
Resultados: el pretratamiento con Achyrocline satureioides en todos
los grupos revirtió el déficit funcional, y la decocción
durante 21 días también decreció de modo significativo
el volumen del infarto. La tinción de Nissl mostró alto porcentaje
de población neuronal conservada y el fluoro Jade presentó un
decrecimiento en las neuronas en degeneración.
Conclusiones: los niveles de quercetina en la decocción y el plasma
de las ratas podrían explicar los beneficios preventivos de Achyrocline
satureioides, debido a las propiedades antioxidantes y antiinflamatorias
descritas para este flavonoide.
Palabras clave: Achyroclines satureioides, quercetina, isquemia focal permanente.
INTRODUCTION
Cerebrovascular attacks (CVA) are the second most frequent cause of death world-wide and the third cause of adult disability in the United States.1 The ischemic CVA (ischemic stroke) results from a transient or permanent reduction in blood flow that affects the territory of a brain artery and accounts for approximately 80 % of all CVA;2 however, and in spite of this high incidence, there is only one FDA-approved therapy -the thrombolytic tissue plasminogen activator (tPA)- for the acute treatment of ischemic CVA.3 Ischemia initiates a complex cascade of events, including increased excitotoxicity, loss of membrane permeability with accumulation of intracellular Ca2+, activation of degradative enzymes, activation of inflammatory signals, etc.4 Oxidative stress is a common final path that could cause lipoperoxidation, nuclear DNA damage and neuronal death5,6 as well as the production of inflammatory mediators.7
These molecular mechanisms that occur during the ischemic cascade have promoted the search of antioxidant and antiinflammatory molecules that could interfere with the oxidative stress, reducing neuronal damage.8 Due to their high antioxidant and antiinflammatory actions, early evidences pointed out to plants, fruits, beverages like wine and tea, and their main compounds such as flavonoids, as meaningful candidates in the search for neuroprotective principles.9
Diverse populations have utilized plants extracts to treat neurological diseases.10,11 People of South America used regularly plants extracts for their neurological effects as Erythroxylon coca (coca) 12 or Chondodendron tomentosum (curare).13 In this context, the enormous variety of native plants of the South American region has been very poorly studied on their value in the prevention of nervous system diseases. The search for pharmacological actions useful in CVA is certainly a meaningful endeavor.14
In a large Southern South America region that covers Argentina, Uruguay, Brazil and Paraguay there are numerous plants with a great arsenal of molecules with antioxidant capacity. In particular Achyrocline satureioides (Lam.) DC. (marcela) is a plant of popular use in this region whose decoctions or infusions have been traditionally used for gastrointestinal disorders, as a sedative and antispasmodic.15 The antioxidant capacity and free radical scavenging of Achyrocline satureioides (AS) has been demonstrated in diverse experimental models16,17 and it was reported that AS protected cells in culture against an oxidative insult.18 Furthermore, it has been shown that the aqueous extracts of AS is not toxic in a maxima tolerable dose of 5 g/kg.19
Phytochemical analysis of AS shows that its main compounds are polyphenols and flavonoids as: caffeic acid, esters of galangin-3-methyl ether, quercetin;20 luteolin;21 3-methoxy-quercetin22 and a new chalcone: achyrobichalcone23 among many other compounds.24 In this regard, the beneficial effect of AS has been ascribed to its content in flavonoids like quercetin,9,17 a molecule that has been shown to be neuroprotective in several models in vitro5 and in vivo, when delivered in a liposomal preparation after focal ischemia in rat.6,25 However, there are no reports of the neuroprotective effect of AS preparations in vivo.
Taking into account the non-toxicity and high popular consumption of this plant in South America, we decided to explore the putative benefits of a chronic oral pre-treatment with an AS decoction in a model of permanent middle cerebral artery occlusion (pMCAo) in rats. We evaluated the behavioral neurological deficit and the cerebral infarct volume of the rats pre-treated with the decoction and subjected to pMCAo. A histopathological examination using Nissl-staining and the fluorescent marker for neuronal degeneration Fluoro-Jade26 was conducted. Given the numerous evidences showing the neuroprotective capacity of quercetin5,8,25,27 and being this flavonoid a conspicuous component of AS,17 it appeared worthwhile to assess the presence of quercetin in the decoction and its bioavailability in the plasma of the experimental animals to assess the possibility of being the responsible of AS effects.
METHODS
Chemicals and reagents
Ketamina (Vetanarcol), Xylacina-HCl (Vetcross), TTC (2,3,5,-triphenylltetrazolium chloride), and HEPES were obtained from Sigma (St. Louis, MO, USA). Fluoro-jade was obtained from Chemicon International, USA. Other chemicals were of highest commercially available purity and were purchased from Baker (Phillipsburg, PA, USA).
Plant material
Achyrocline satureioides (Asteraceae) was obtained from Institute of Agriculture Research (INIA) "Las Brujas", Canelones, Uruguay. The species were identified by Ing. Agr. P. Davies. A voucher specimen of Achyrocline satureioides was kept in the College of Agronomy Republic University, Montevideo, Uruguay (MVFA 32796).
Decoction preparation
Two grams of Achyrocline satureioides dried flowers were added to 100 mL of boiling water that was kept boiling for 30 min. After that, the decoction was allowed to reach room temperature for 3 hours when the flowers were drained and separated from the decoction.
Animals and experimental protocol
Experiments were carried out using male Sprague-Dawley rats (280-350 g). Animals had access to food and water ad libitum, and were housed in groups of six in a temperature controlled environment (22 ± 3 °C) under a 12 h light/dark cycle. The experiments on animals were approved by the Bioethics Committee of Animal Care from Clemente Estable Institute: Protocol No. 002/5/2010. In some animals water was replaced with the AS decoction during 7, 14, or 21 days (n= 5/groups). The quercetin contained in the AS decoction was evaluated weekly by HPLC.
For behavioral and morphometric studies (Infarct volume) thirty six animals were divided into six groups: Group 1: AS decoction during 7 days; Group 2 during 14 days; Group 3 during 21 days (n= 5/groups) all followed by pMCAo during 24 hours. Group 4: pMCAo during 24 h. (n= 9). Group 5: AS decoction only during 7, 14 and 21 days (n= 3/groups). Group 6: sham operated (n= 3). In a second set of rats, the parietal cortex and striatum of the animals were used for histological and histochemical analysis: Nissl (n= 4) and Fluoro-Jade (n= 6).
The body weight of the all animals was assessed weekly and the controls of the intake of food and AS decoction and/or water (in the case of the control animals) were carried out daily.
Behavioural testing
Twenty four hours after pMCAo, and previous to the sacrifice, the animals of all experimental groups were subjected to a neurological examination as described by Menzies et al.28 Briefly, the behavior of animals was scored as 0: no apparent deficits; 1: contra-lateral forelimb flexion when suspended by the tail; 2: decreased grip of the contra-lateral forelimb while tail pulled; 3: spontaneous movement in all directions, contra-lateral circling only if pulled by tail and 4: spontaneous contra-lateral circling. Tests were conducted blinded.
Ischemic surgery
Control and experimental animals were anaesthetized with ketamine (80 mg/kg) and Xylazine (5 mg/kg), anesthetics frequently utilized in ischemic experimental procedures.29,30 Since there are some reports of a neuroprotective action of ketamine,31 a sham operated group was performed to control this putative effect on the global final assessment of protection. Body temperature of the animals was continuously monitored throughout the surgical procedure with a rectal thermometer, and maintained at 37.5 °C with a heating pad. The focal cerebral ischemia was induced by pMCAo model as described by Sydserff and co-workers32 with minor modifications. In brief, a surgical midline incision was made to expose the left common, internal and external carotid arteries. The external carotid and the common carotid arteries were closed by a ligature, the occipital artery was cut by diathermy using a coagulator and the internal carotid artery was temporarily occluded using a micro-aneurysm clip. A small incision was then made in the common carotid artery, and a 19-mm length of 4-0 monofilament nylon sutures, its tip rounded by heating, was introduced into the internal carotid artery. This tip is soft and flexible and reduces the risk of vessel injury and intracranial bleeding. The filament was advanced up to the appearance of a mild resistance to this advancement indicating that the intraluminal occluder has entered the anterior cerebral artery and occluded the origin of the anterior cerebral artery, the middle cerebral artery (MCA) and posterior communicating arteries.33 The filament was left in place, fixed to the left carotid artery, at its incision point of entrance by a diathermic point using a coagulator. Skin was finally closed with four suture points with regular suture thread. The filament was in place for the next 24 h. In the groups of sham-operated rats all surgical procedures except the pMCAo were performed. The animals were then allowed to recover from the anesthesia and returned to their cages after the surgery with free access to food and water.
Quantification of the cerebral infarct volume
Twenty-four hours after the pACMo, the animals of all experimental groups were re-anaesthetized with urethane (1.2 g/mL) and intracardially perfused with 0.9 % NaCl (200 mL).34 Brains were quickly dissected and sectioned at 2, 5, 8, and 11 mm from the frontal lobe. Five coronal sections from each brain (from 2.20, 1.20, 0.20, -0.26, and -1.30 mm with respect to Bregma35 were obtained. After sectioning, slices were incubated for 30 min in a 2 % solution of TTC in 0.9 % NaCl at 37 °C and then fixed in 10 % formalin.36 The stained slices were photographed with a digital camera and the zones of infarction and brain total areas were outlined.6,37 These areas were quantified by the image processing program, (Image Pro Plus) and the total infarct volume was calculated by integrating the infracted area of all sections (area of infarct in mm2 x section thickness). To compensate for swelling, the following formula was applied: infarct size x contralateral hemisphere size/ipsilateral hemisphere size, according to Chan et al.38 Results were expressed as infarct volume in mm3.
Processing of the tissue
The animals treated during 21 days with the AS decoction and within 24 hours of the pMCAo, were anesthetized; the brains were perfused through the left heart ventricle with heparinised saline solution (200 mL), followed by 4 % paraformaldehide (300 mL). After that, a cryoprotection procedure with 15 and 30 % sucrose (w/v) was carried out. The brains were rapidly frozen for 3 min afterwards and coronal brain cryosections (20 µm) were serially cut.39
Nissl stain
The sections were mounted on slides, washed three times for 5 min with PBS-Triton X-100 (0.4 %) and subsequently stained with Toluidine blue 0.1 %, for approximately 2 min. Finally, dehydration of the sections was done and mounted with Canada balsam. In order to correct for edema differences in brain volumes in each experimental group, stereological procedures were used.40 For this purpose a homothetic transformation of areas in tissue sections of each group was performed.
In order to asses the effects of AS treatment on the survival of the neurons in striatum, in sections ipsilateral to the occluded medial cerebral artery (0.20 µm with respect to Bregma), photographs of 4 fields adjacent to the lateral ventricle were taken with a Nixon microscope (Magnification 20X). On the photographs, the total population of survival neurons (those with echromatic nucleus and conserved cytoplasm in the 4 experimental groups (Sham; AS; Ischemia and treatment 21 days AS + ischemia) (n= 4/group) were quantified. Number of neurons on sham operated animals was taken as 100 % and the percentage of viable cells was quantified accordingly.
Histochemistry of Fluoro-Jade
Degenerating neuronal somata and their processes were detected with Fluoro-Jade staining as originally described by Schmued et al.41 Brain sections were serially incubated in the following solutions for the time indicated: 100 % alcohol, 3 min; 70 % alcohol, 1 min; distilled water, 1 min; 0.06 % potassium permanganate, 15 min; H20, 1 min; 0.001 % Fluoro-Jade in 0.09 % acetic acid, 30 min; H20, 2 x 1 min. Stained sections were allowed to dry at room temperature protected from light and mounted with Canadian Balsam. Sections were examined using an Olympus IX81 fluorescent microscope equipped with a DP71 Olympus camera.
Quercetin quantification
To evaluate the stability of the decoction of AS, quercetin concentrations were assessed weekly according to a standard procedure in a Waters modular HPLC system (Waters Associates, Milford, MA.).42 Separation of constituents was achieved by reverse-phase HPLC using a C18 column (Phenomenex,USA) with 5 mm particle size. A binary HPLC pump (Waters 1525) with a 717 plus autosampler Waters and a photodiode array detector Waters 2998 linked to Empower 2 (Waters) chromatography data software was utilized. The temperature of the column was set at 30 °C. The mobile phase used was: (A) 100 % MeOH, (B) 0.5 % H3PO4 pH= 2, 5 % MeOH, at 0.7 mL/min. The gradient system consisted of (min/%B): 0/80, 40/0, 41/80, 47/80. The eluant was monitored by photodiode array detection at 375 nm and spectra of products obtained between 210-600 nm.
Plasma sample preparation
Control rats treated with decoction during 7, 14, and 21 days (n = 2/groups) were anesthetized with urethane (2.4 g/kg) and whole blood sample was obtained from the left ventricles, centrifuged at 1500 rpm, for 15 min at 4 °C and supernatants were injected in a Waters modular HPLC system using the same protocol mentioned above.
Statistical analysis
All data are presented as mean ± SD. Data on the behavior, infarction volume and cellular counts were statistically analyzed and the differences between the means of different experimental groups were analyzed by one way analysis of variance (ANOVA) followed by Tukey-Kramer test for multiple comparisons.* Statistical significance was accepted at p< 0.05.
RESULTS
There was no difference in the daily volume intake of the decoction or water neither in weekly body weight increase in experimental and control animals along the experiment. During the first week of treatment animals receiving AS showed a significant increase in food intake that was not reflected in body weight increases and lasted only one week (data not shown).
Neurological test
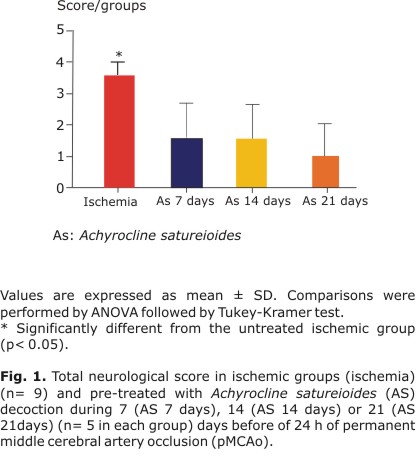
Before the pMCAo the neurological score was normal (score = 0) in all animals according to the procedure of Menzies et al.28 Vehicle-treated ischemic rats presented scores corresponding to severe behavioral impairments 24 h after pMCAo (3.4 ± 0.5) (Fig. 1 - Ischemia). The animals pre-treated with the AS decoction during 7 (AS 7 days), 14 (AS 14 days), and 21 (AS 21 days) days before the permanent occlusion showed a significantly improvement in neurobehavioral performance compared to vehicle treated rats: (1.4 ± 1.1); (1.4 ± 0.9), and (1.2 ± 1.1), respectively (Fig. 1).
Cerebral Infarct Volume
The infarct volume assessed after 24 h of pMCAo was reduced in the ischemic group pre-treated with AS during 21 days (6.9 ± 1.3 mm3) compared to the ischemic group (Ischemia) that took only water (10.9 ± 3.2 mm3). The animals pre-treated during 7 (AS 7 days) and 14 days (AS 14 days) with AS showed infarct volume values similar to the ischemic group: 9.2 ± 1.8 mm3 and 8.8 ± 1.9 mm3 respectively, (Figs. 2 and 3). No lesion was observed in the groups without pMCAo (control, AS decoction for 7, 14 and 21 days) and in sham operated animals (data not shown).
Nissl staining
The figure 4 shows the neuronal population in the striatum of the different experimental groups, stained with Nissl. Striatal neurons of the control animals (Fig. 4A, [Sham], Fig. 4C [As]) showed a conservation of the general histoarchitecture of the white/grey matter, with neurons with round body and clear cytoplasm with Nissl granules plus defined nucleus.
In ischemic animals (Fig. 4B), there is an almost complete loss of tissue histo-architecture that does not allow even to differentiate the white matter from the surrounding parenchyma. Neurons appear hyperchromatic and have lost the round form of normal ones and phantom nuclei appear scattered in the field.
As it can be observed in figure 4D, the global appearance of the tissue in ischemic groups pre-treated with AS during 21 days was closer to normal (Fig. 4A) with apparent increased number of normal neurons compared to ischemic rats. Nevertheless, general histoarchitecture of the white/grey matter difference is not observed (Fig. 4D). The striatal sections of animals treated with the AS decoction, and no ischemia, did not show evident damage (Fig. 4C).
When the number of striatal neurons with normal structural features were counted in every experimental group and compared with the number of neurons in sham operated animals (Fig. 5), a significant reduction in the number of cells was observed in the ischemic group (50 %) (Fig. 5, [Ischemia]). A significant recovery in the neuronal population was observed with AS pre-treatment during 21 days (80 %) (Fig. 5, [AS + Ischemia]).
Fluoro-Jade staining
Since the Fluoro-Jade technique is a neuron-degenerating staining, striatal sections of controls animals show no reactivity. In contrast, the ischemic striatum showed marked staining indicating degenerating neurons. Fewer reactive neurons were seen the groups pre-treated with AS during 21 days). Reactivity is not present in the AS treated groups without ischemia (data not shown).
Quercetin quantification in decoction and plasma of rats
Quercetin concentration in the decoction (mg/mL) measured by HPLC was of 1.14 ± 0.19 mg/mL and showed a significant stability during the 21 days of the experiment. Every rat showed an intake of 43.6 ± 6.64 mL which were equivalent to 50.2 ± 17.9 mg/mL of flavonoids expressed as equivalents of quercetin. Quercetin levels in the rat plasma was 60.4 ± 58.2 ng/mL) after 21 days of administration of the decoction. Treatment alone did not show differences with sham-operated animal.
DISCUSSION
Results obtained in the present study give, for the first time, consistent evidence for a neuroactive capacity of aqueous preparation of AS when administered chronically, in the lesions provoked by permanent focal ischemia in the rat. The decrease in the extension of the lesion demonstrated by TTC, the decrease of degenerative neurons shown by Fluoro-Jade, the increase in the number of surviving neurons and the recovery of motor behaviour are a clear support for the protective capacity.
Importantly, no significant changes between the control animals and the AS- treated group in terms of the increase of body weight or intake of food and decoction were quantified and the preparation was well accepted by the experimental animals. No significant effects on either of the assessment procedures utilized were observed. Additionally, another important distinctive aspect of the present study is the chronicity of the intake of the decoction. Several studies on the effects of plants or even isolated flavonoids have shown that much better bioavailability can be obtained after prolonged administration.43 An addtional strenght of these results is that they are obtained in the striatum, the brain region most affected in the experimental pMCAo.8 Previous studies and our own results (Fig. 2) have shown that other brain areas reached by the main artery occluded, the medial cerebral artery, such as the parietal cortex, are less lesioned.37 This could be an explanation for the apparent discrepancy between the histological and motor behavioral results. The parietal cortex, less lesioned, could get a quicker recovery as shown by improvement in motor deficits.
Morphometric analysis of the infarct volume using TTC staining has been commonly employed to determine the efficacy of cerebroprotective compounds in pre-clinical trials. TTC is a sensitive histochemical indicator of mitochondrial respiratory enzyme function.44 Therefore, brain lesion identified by TTC staining indicates that mitochondrial function and oxidative respiratory enzyme systems in those tissues are impaired.6,37 Figure 2 showed a possible influence of the AS pretreatment during 21 days in the neuronal oxidative system.
With these results, AS would join the selected group of plant with demonstrated action on the central nervous system. Only a small group of plants are recognized in Western world as neuroactive, including Ginkgo biloba, St John's wort, Kava-kava, Valerian, Bacopa monniera and Convolvulus pluricaulis. Nevertheless, with the exception of Gikngo biloba and likely Bacopa monniera10 most of them are effective in anxiety and insomnia and not in protective or anti neurodegenerative actions.45-47
Although AS is not utilized popularly for nervous system ailments, previous studies in cells in culture have already shown its protective capacity.18 Other studies, including data from our group, show that the flavonoids quercetin, luteolin and 3-O-methylquercetin are the main chemical constituents of the AS preparations9,16,18 and precisely; quercetin and structural related flavonoids are neuroprotective. Our group was the first to demonstrate that there are structural features of flavones specifically related to the neuroprotection.48 It is then very likely that the results on neuroactive capacity exerted by AS decoction could be at least partially explained by the actions49,50 ascribed to its constituent flavonoids: quercetin and luteolin.17 Studies in vivo describe the neuroprotective effects of the luteolin in the ischemia models in rats51,52 and addition to our group6,25 recent experimental evidence confirms the neuroprotective effects of quercetin against cerebral ischemia.53
Ishisaka et al., using High Performance Liquid Chromatography (HPLC) and mass spectrometry showed that the quercetin can accumulate enough to exert biological activity in rat brain during a chronic oral administration.54 Chronic flavonoids intake in the diet or after use of plants with ethno-pharmacological profile could reach pharmacological levels in the brain by an accumulative effect.55 However the mechanisms globally supporting the beneficial effects of these flavonoids in the brain in vivo remain to be elucidated. Our result showed that plasma bioavailability of quercetin was increasing along the experiment and it is likely that the same happens in the brain. Nevertheless, these aspects together with experiments with a neutral fraction (flavonoid) of AS are part of our present work, which is intended to provide more data on the role played by flavonoid in the observed effects. Taking into account the broad popular use of AS, there is no toxicity described in experimental study and its high content of flavonoids, makes possible to propose that a chronic oral administration with AS preparation may offer preventive benefits in people aged and with risk of stroke.
ACKNOWLEDGMENTS
The authors wish to thank the optical facilities at Cell Biology and Molecular Department of the Science Faculty, Republic University and its head, Dr. Cristina Arruti. Also the authors wish to thank MSc. Andrea Toledo, MSc. Carolina Echeverry and student Vicente Ruiz for their contributions and technical assistance in this manuscript.
REFERENCES
1. Feigin VL. Stroke epidemiology in the developing world. Lancet. 2005;365(9478):2160-1.
2. Rosamond K, Flegal K, Purie E. Heart disease and stroke statistics- update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2008;117(4):e25-e46.
3. Armstead K, Ganguly JW, Kiessling W. Signaling delivery and age as emerging issues in the benefit/risk ratio outcome of tPA for treatment of CNS ischemic disorders, J Neurochem. 2010;113(2):303-12.
4. Clark WM, Rinker LG, Lessov NS, Lowery SL, Cipolla MJ. Efficacy of antioxidant therapies in transient focal ischemia in mice. Stroke. 2001;32(4):1000-4.
5. Dajas F, Rivera F, Blasina F, Arredondo F, Echeverry C, Lafon L, et al. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotoxicity Research. 2003;5(6):425-32.
6. Rivera F, Urbanavicius J, Gervaz E, Morquio A, Dajas F. Some aspects of the in vivo neuroprotective capacity of flavonoids: Bioavailability and structure-activity relationship. Neurotoxicity Research. 2004;6(7-8):543-53.
7. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transi Med. 2009;7:97 doi: 10.1186/1479-5876.
8. Rivera F, Costa G, Abin A, Urbanavicius J, Arruti C, Casanova G, et al. Reduction of ischemic brain damage and increase of glutathione by a liposomal preparation of quercetin in permanent focal ischemia in rats. Neurotoxicity Research. 2008;13(2):105-14.
9. De Souza KCB, Bassani VL, Schapoval EES. Influence of excipients and technological process on anti-inflammatory activity of quercetin and Achyrocline satureioides (Lam.) D.C. extracts by oral route. Phytomedicine. 2007;14(2-3):102-8.
10. Jyoti A, Sharma D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology. 2006;27(4):451-7.
11. DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA. Ginkgo biloba for prevention of demencia: a randomized controlled trial. JAMA. 2008;300(19):2253-62.
12. Biscoping J, Bachmann-Mennenga MB. Local anesthetics from ester to siomer. Anasthesiol Intensivmed Notfallmed Schmerzther. 2000;35(5):285-92.
13. Reynolds T. Hemlock alkaloids from Socrates to poison aloes. Phytochemistry. 2005;66(12):1399-406
14. Lloyds-Jones D, Adams RJ, Brown TM. Heart disease and stroke statistics-2010 update: A report from the American heart association. Circulation. 2010;121(12):e46-e215.
15. Filot Da Silva L, Langeloh A. Comparative activity of hydroalcoholic 80 % (v/v) of Achyrocline satureioides (Lam.) DC (Asteraceae) with papaverine and atropine on rat isolated jejunum. Acta Farmaceutica Bonaerense.1994;13:35-40.
16. Desmarchelier C, Coussio J, Ciccia G. Antioxidant and free radical scavenging effects in extracts of the medicinal herb Achyrocline satureioides (Lam.) DC (AS). Braz J Med Biol Res. 1998;31(9):1163-70.
17. De Souza KCB, Schapoval EES, Bassani VL. LC determination of flavonoids: separatin of quercetin, luteolin and 3-0-methylquercetin in Achyrocline satureioides preparations. J Pharm Biomed Anal. 2002;28(3-4):771-7.
18. Arredondo MF, Blasina F, Echeverry C, Morquio A, Ferreira M, Abín Carriquiry JA, et al. Cytoprotection by Achyrocline satureioides (Lam.) D.C. and some of its main flavonoids against oxidative stress. J Ethnopharmacol. 2004;91(1):13-20.
19. Rivera F, Gervaz E, Sere C, Dajas F. Toxicological studies of the aqueous extract from Achyrocline satureioides (Lam.) DC (AS). J Ethnopharmacol. 2004;95(2-3):359-62.
20. Dellacassa E, Ferreira F, Menéndez P, Moyna P, Rossini C, Soule S, et al. Diferencias varietales em los flavonoides de Achyrocline satureioides (Lam.) DC. In: II Congreso de Ciências Farmacêuticas del Cono Sur, Montevideo, Uruguay; 1993.
21. Petrovick PR, Knorst M. Efeito da operacao son pressoa reducida sobre a qualidade dos extratos concentrados de Achyrocline satureioides (Lam.) DC. Compositae. In: Abstracts ot the XII Simpósio de plantas medicinais do Brasil. Curitiba; 1992.
22. Simoes CMQ, Petrovick P, Bassani P. Analyxix of flavonoids from Achyrocline satureioides (Lam.) DC. Compositae. Rev SAFYBI. 1988;28(3):2626-30.
23. Holzchuh MH, Gosmann G, Schneider PH, Schapoval EES, Bassani VL. Identification and stability of a new bichalcone in Achyrocline satureioides spray dried powder. Pharmazie. 2010;65(9):650-6.
24. Retta DS, López PG, Gattusso MA, Gattuosso SJ. Optimizatiohon ahdn validatiaoan of the quantiative assay of flavonoids in Achyrocline satureioides and A. flaccida. Lat Am J Pharm. 2011;30(7):1360-5.
25. Dajas F, Rivera F, Blasina F, Arredondo F, Abín-carriquiry JA, Costa G, et al. Neuroprotection by flavonoids. Braz J Med Biol Res. 2003;36(5):1613-20.
26. Allen GV, Gerami D. Conditioning effects of repetitive mild neurotrauma on motor function in an animal model of focal brain injury. Neuroscience. 2000;99(1):93-105.
27. Hwang IK, Lee CH, Yoo KY. Neuroprotective effects of onion extract and quercetin against ischemic neuronal damage in the gerbil hippocampus. J Med Food. 2009;12(5):990-5.
28. Menzies SA, Hoff JT, Betz H. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31(1):100-7.
29. Yonggang L, Zhenfeng X, Gregory D. Neuroprotection by Neuregulin-1 et al. Neuroprotection by Neuregulin-1 in a rat model of permanent focal cerebral ischemia. Brain Research. 2007;1184(4):277-83.
30. Zhao H, Mayhan WG, Sun H A. Modified suture technique produces consistent cerebral infarction in rats. Brain Research. 2008;30(1246):158-66.
31. Sakowitz OW, Kiening KL, Krajewski KL. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke. 2009;40(8):519-22.
32. Sydserff SG, Cross AJ, Green AR. The neuroprotective effect of chlormethiazole on ischaemic neuronal damage following permanent middle cerebral artery ischaemia in the rat. Neurodegeneration. 1995;4(3):323-8.
33. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84-91.
34. Preston E, Webster J. Spectrophotometric measurement of experimental brain injury. J Neuroscience Methods. 2000;94(2):187-92.
35. Paxinos G, Watson C. The Rat Brain Stereotaxic Coordinates. 3rd ed. San Diego: Academic Press; 1985. p. 375-415.
36. Yu SJ, KIM JR, Lee CK, Han JE, Lee JH, Kim HS. Gastrodia elata Blume and an active component, p-Hydrobenzyl alcohol reduce focal ischemic brain injury through antioxidant related gene expressions. Biol Pharm Bull. 2005;28(6):1016-20
37. Belayev L, Yitao L, Weizhao Z, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke. Stroke. 2001;32(2):553-65.
38. Chang ML, Yang J, Kem S, Klaidman L, Sugawara T, Chan PH, et al. Nicotinamide and ketamine reduc infarct volume and DNA fragmentation in rats after brain ischemia and reperfusion. Neuroscience Letters. 2002;322(3):137-40.
39. Storini C, Bergamaschini L, Gesuete R, Rossi E, Maiocchi D, De Simoni G. Selective inhibition of plasma Kallikrein protects brian from reperfusion injury. J Pharmacol Experimental Therapeutics. 2005;318(2):849-54.
40. Raghanti MA, Spocter MA, Stimpson CD, Erwin JM. Species-specific distributions of tyrosine hydroxylase-immunoreactive neurons in the profrontal cortex of anthropoid primates. Neuroscience. 2009;158(4):551-9.
41. Schmued LC, Slikker AC. Fluoro-Jade: a novel fluochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Research. 1997;751(1):37-46.
42. Arredondo F, Echeverry C, Abin-Carriquiry JA, Blasina F, Antúnez K, Jones DP, et al. After celular internalization quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic Boil Med. 2010;49(5):738-47.
43. Datla KP, Christidou M, Widmer WW. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretinin a rat model of Parkinson´s disease. Neuroreport, 2001;12(17):3871-5.
44. Bederson JB, Pitts LH, Germano RL, Nishimura D, Bartowski HM. Evaluation of 2, 3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17(6):1304-8.
45. Kumar V. Potential Medicinal Plants for CNS Disorders: an Overview. Phytother Res. 2006;20(12):1023-35.
46. Bachinskaya N, Hoerr R, Ihl R. Alleviating neuropsychiatric symptoms in dementia: the effects of Ginkgo biloba extract EGb 761. Findings from a randomized controlled trial. Neurospsychiatr Dis Treat. 2011;7doi: 10.2147/NDT.S18741:209-15.
47. Dajas F, Abín A, Arredondo F. The neuroprotective capacity of Achyrocline satureioides (Lam) D.C. and its flavonoids. Mechanisms of action. In: G. Bagetta MI, Corasanti M, Cosentino S, editors. Sakurada (Org.) HERBAL FOR HUMAN HEALTH: Development and Evaluation of Plant-derived medicines. v.1. Londres; Taylor and Francis; 2010. p. 339-49.
48. Echeverry C, Arredondo F, Abin-Carriquiry JA. Pretreatment with natural flavones and neuronal cell survival after oxidative stress: A structure-activity relationship study. J Agriculture Food Chemistry. 2010;58(4):2111-5.
49. Pauff JM, Hille R. Inhibition studies of bovine xanthine oxidase by luteolin, silibinin, quercetin, and curcumin. J Nat Prod. 2009;72(4):725-31.
50. Pandey AK, Hazari PP, Patnaik R, Mishra AK. The role of ASIC1a in neuroprotection elicited by quercetin in focal cerebral ischemia. Brain Res. 2011;6(1383):289-99.
51. Zhao G, Zang SY, Jiang Z H, Chen YY. Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J Nutr Biochem. 2011;22(10):929-36.
52. Qiao H, Dong L, Zhang X, Zhu C, Zhang X, Wang L, et al. Protective effect of Luteolin in experimental ischemic stroke_ Upregulated SOD1, CAT, Bcl-2 and Claudin-5, down-regulated MDA and Bax expression. Neurochem Res. 2012;37(9):2014-24.
53. Ahmad A, Khan M, Hoda MN, Raza SS, Khan MB. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem Res. 2011;36(8):1360-71.
54. Ishisaka A, Ichikawa D, Sakakibara H, Mariusz K, Nakamura T. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radical Biology Medicine. 2011;51(7):1329-36.
55. Liu J, Oiao W, Yang Y, Ren L, Sun Y, Wang S. Antidepressant-like effect of the ethanolic extract from Suanzaorenhehuan Formula in mice models of depresion. J Ethnopharmacol. 2012;141(1):257-64.
Recibido: 18 de diciembre de 2012.
Aprobado: 17 de mayo de 2013.
Felicia Rivera Megret. Department of Neurochemistry. Instituto de Investigaciones Biológicas Clemente Estable. Avda. Italia 3318, 11600 Montevideo, Uruguay. Telephone: 598 2 487 1616 int. 123. Fax: 598 2 487 26 03. E-mail: frivera@iibce.edu.uy; riveramegret@gmail.com