Rev Cubana Plant Med. 2016;21(3)
ARTÍCULO ORIGINAL
Antimicrobial activity of essential oil from Baccharis trimera (Less.) DC. (carqueja-amarga)
Aceite esencial de Baccharis trimera (Less.) DC. (carqueja-amarga): actividad antimicrobiana
Érika Yoko Suzuki, César Augusto Caneschi, Romário Costa Fochat, Marcos Antônio Fernandes Brandão, Nádia Rezende Barbosa Raposo
Faculdade de Farmácia, Universidade Federal de Juiz de Fora, Juiz de Fora-MG, Brazil.
Financial support
The authors acknowledge the financial support from CAPES.
ABSTRACT
Introduction:
the recent enhancement of interest in 'green' consumerism has given rise
to a renewed scientific awareness towards essential oils. Essential oil
from Baccharis trimera (Less.) DC. (B. trimera) (Asteraceae) is
cited as one of the ten most consumed oils by the cosmetic and other industries
in Brazil.
Objective:
to investigate the antimicrobial activity of the essential oil from the
leaves of B. trimera against Staphylococcus epidermidis ATCC 12228,
Proteus vulgaris ATCC 13315, Micrococcus luteus ATCC 7468 and
Corynebacterium xerosis IAL105, which are the main bacteria responsible
for bad perspiration odor.
Methods: the gas chromatography (GC) analysis was performed and the antimicrobial
activity was evaluated by means of the turbidimetric method, using a microdilution
assay.
Results:
ywenty constituents were identified, being that β-pinene (23.4%) was the
major compound found. The minimum inhibitory concentration (MIC) values of the
essential oil ranged from 500 μg/mL to 1,000 μg/mL. A detrimental
effect of the essential oil was observed on the morphology of cell membranes
of the bacteria studied by scanning electron microscopy (SEM).
Conclusions:
yhe results demonstrate the essential oil of B. trimera has potential
in the application of antimicrobial agents in personal care products.
Key words: Baccharis; bacteria; medicinal plants; personal hygiene products; products with antimicrobial action; scanning electron microscopy.
RESUMEN
Introducción:
el reciente aumento del interés por el consumo "verde" ha dado lugar
a una renovada conciencia científica hacia a los aceites esenciales. El
aceite esencial de Baccharis trimera (Less.) DC. (B. trimera)
(Asteraceae) es considerado uno de los diez aceites más consumidos por
la industria cosmética del Brasil.
Objetivos:
valorar la actividad antimicrobiana del aceite esencial de hojas de B. trimera
frente al Staphylococcus epidermidis ATCC 12228, Proteus vulgaris
ATCC 13315, Micrococcus luteus ATCC 7468 y Corynebacterium xerosis
IAL105, que son las principales bacterias responsables del mal olor que es consecuencia
de la transpiración.
Métodos:
se realizó la cromatografía de gases (GG) y la actividad antimicrobiana
fué valorada por el método turbidimétrico, usando el ensayo de
microdilución.
Resultados:
se identificarón veinte constituyentes, siendo el β-pineno (23,4%)
el principal compuesto encontrado. Los valores de la concentración mínima
inhibitoria (CMI) del aceite esencial variaron de 500 μg/mL a 1,000 μg/mL.
Se observó un efecto perjudicial del aceite esencial en la morfología
de las membranas celulares de las bacterias estudiadas por microscopía
electrónica de barrido (SEM).
Conclusión:
los resultados demuestran que el aceite esencial de B. trimera tiene
potencial en la aplicación de los agentes antimicrobianos en productos
de higiene personal.
Palabras Clave: Baccharis; bacterias; plantas medicinales; productos de higiene personal; productos con acción antimicrobiana; microscopía electrónica de barrido.
INTRODUCTION
Many people use numerous personal care products between them deodorant, which are carefully prepared by using intricate recipes and a variety of substances with diverse functions. Such substances include active ingredients, solvents, preservatives and additives, some of which are suspected to affect the consumer's health, such as phthalates, parabens or antimicrobials (triclosan/triclocarban).1
Deodorants deserve special attention, in its formulation including antimicrobial substances and fragrances whose aim to control the bacterial growth and avoid bad body odor caused by them. According to Euromonitor International, the global deodorant market stood at almost $20.4 bn in 2011, up 6.7% from $19.1 bn in 2010. The deodorant sector is on the rise both in active growth as saturated markets due to innovative of the targeted products, such as Latin America, Pacific Asia, the Middle East, and Africa were major growth areas.2
Essential oils are frequently incorporated into these kinds of products because of the complexity of their active compounds, strong fragrant properties and 'natural' marketing image.3,4 Essential oils are present in almost 3,000 different species of plants, 10% of this total being of commercial interest in agriculture, food industry and the pharmaceutical area.5
Although some essential oils have long been researched for their medicinal properties, the recent enhancement of interest in 'green' consumerism has given rise to a renewed scientific awareness towards them.6 The growing 'green' consciousness is pushing to the consumers to choose premium natural personal care products, instead of products containing synthetic ingredients. Natural cosmetics exhibited an increased revenue of 20.9%, partly as a result of the increased awareness towards the chemicals contained in some commercial products.7 Moreover, essential oils have shown activity as penetration enhancers for antiseptics and could be applied as antimicrobial actives against antiseptic-resistant species.8
Among the different species of the plants studied, Baccharis trimera (Less.) DC. (Asteraceae) is native to South America and it is popularly known as "carqueja-amarga". The leaves of B. trimera are widely used in the popular medicine in the form of infusion in order to obtain an anti-inflammatory, antacid, antiulcer and digestive extract.9-12 The hydroalcoholic extract of the leaves and flowers of the plant also shows potential as an antioxidant and an antiulcer,13 and it is used in the treatment of rheumatism, hepatobiliary disorders,14 diabetes, as well as an antimicrobial,15 antifungal16 and antigenotoxic agent.14 The essential oil extracted from the shrub presents activity against Schistosoma mansoni11 and Candida albicans.17 It is currently employed in the food and in the pharmaceutical industries.11 Additionally, it has been reported antimicrobial activity of the essential oil obtained from aerial parts of many species of Baccharis gender indicating action against Gram-negative and Gram-positive bacteria.18-22 Moreover, essential oil from B. trimera is cited as one of the ten most consumed oils by the cosmetic and other industries in Brazil.15
Based on this context, the aim of this work was to evaluate the antimicrobial activity of the essential oil of B. trimera against four main bacteria responsible for bad perspiration odor, as a possible alternative for application as an antimicrobial agent in personal care products.
METHODS
Essential oil
The essential oil from B. trimera leaves (lot BATRI0111) was obtained from Lazlo Aromatologia Ltda, which was extracted by vapor entrainment.
Gas chromatography
In order to qualitatively and quantitatively characterize the main chemical constituents from essential oil, an aliquot was subjected to analysis by high-resolution gas chromatography (HP 5890) equipped with flame ionization detector. A BP-1 (SGE) 25 m x 0.25 mm column was used, with a temperature gradient of 60°C/1 minute, 3°C/minute to 220°C; injector (split of 1/50) at 220°C and detector at 220°C. The carrier gas used was hydrogen (2 mL/minute) and the injection volume was of 1 μL. Samples were diluted to 0.5% in chloroform. Identification of essential oil components was based on the retention times of sample components and a mixture of n-alkanes from C10-C18 and the calculated Kovats Index compared with the available literature.23 In the biological assays, essential oil concentration is referenced as an equivalent of its major component.
Antimicrobial activity
Microorganisms
Micrococcus luteus (ATCC 7468), Proteus vulgaris (ATCC 13315) and Staphylococcus epidermidis (ATCC 12228) were obtained from the American Type Culture Collection. Corynebacterium xerosis (IAL105) was obtained from Adolfo Lutz Institute Culture Collection.
Antimicrobial screening and minimum inhibitory concentration (MIC)
A total of four microorganisms, including three Gram-positive (Corynebacterium xerosis IAL105, Micrococcus luteus ATCC 7468 and Staphylococcus epidermidis ATCC 12228) and a Gram-negative (Proteus vulgaris ATCC 13315) bacteria were tested. The inhibition of microorganism growth was determined by means of turbidimetric method by using a microdilution assay in a sterile 96-well microplate (Sarstedt, Germany). 24 Each well contained 100 μL of the essential oil (62.5 to 1,000 μg/mL of β-pinene) and 100 μL of Brain Heart Infusion (BHI) for C. xerosis or Mueller Hinton Broth (MHB) for the others with the bacteria representing, approximately, 4 x 103 colony-forming units (CFU)/mL. The microplates were incubated at 35ºC for 24 hours. Next, 30 μL of aqueous solution of 0.01 mg/mL resazurin were added in each well and the microplate was reincubated for 4 hours. The MIC values were determined by change in color, with the highest dilution remaining blue indicating the MIC. In addition, chloramphenicol (0.025 - 250 μg/mL) and neomycin (0.0125 - 125 μg/mL) were used as drugs references. Tests were carried out in triplicate.
Minimum bactericidal concentration (MBC)
The minimum bactericidal concentration value was determined in the wells with absence of growth obtained in the MIC assay and 20 μL of culture of each well were transferred to tubes with Tryptone Soy Broth (TSB). The tubes were incubated at 35ºC for 24 hours. The MBC value was regarded as the lowest concentration of the essential oil where no visible growth was observed.
Scanning electron microscopy analysis
The scanning electron microscopy (SEM) was used to investigate morphological changes in the strains of interest submitted to the treatment with the essential oil, chloramphenicol and neomycin. The SEM was carried out by a method adapted from Gao et al.25 The bacteria cells were incubated for 24 hours in MHB (S. epidermidis, P. vulgaris, M. luteus) or BHI (C. xerosis) at 35ºC. The suspension was treated with essential oil (1,000 μg/mL of β-pinene) or the drugs reference (chloramphenicol and neomycin at MBC value), and then the samples were reincubated at 35ºC for 24 hours. After incubation, cells were harvested by centrifugation for 10 minutes at 5,000 x g and transferred onto slides. The cells were fixed with 2.5% glutaraldehyde for 12 hours. After that, the slides were washed with 0.1 M phosphate buffer solution (pH 7.4), dehydrated with increasing concentrations of ethanol (50 to 100%) with an interval of 20 minutes between each exchange, and dried at room temperature. The slides were assembled in aluminum stubs with double-faced carbon and then covered with gold in a sputter (2 mm) for 2 minutes in Balzers Union FL - 9496 (Balzers, Germany). Subsequently, they were analyzed in the scanning electron microscope JSM 5310 (Jeol, Japan) in high vacuum in secondary electron mode.
RESULTS
Chemical composition of the essential oil
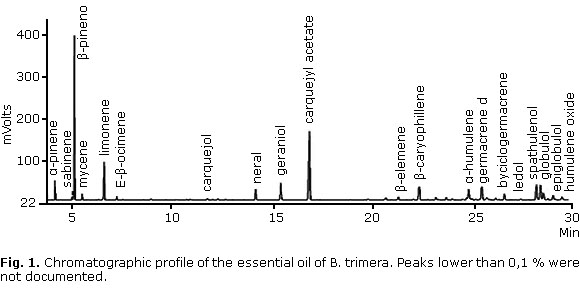
Twenty constituents were identified by GC in the essential oil, accounting for 89.7% of all components in the essential oil. Other components not listed are present in amounts of less than 0.1%. β-pinene (23.4%) and carquejyl acetate (19.0%) were the constituents in highest concentrations (Fig 1 and table 1).
Antimicrobial activity
Minimum Inhibitory Concentration (w/v) and Minimal Bactericidal Concentration (w/v)
According to the results given in table 2, the oil exhibited antimicrobial activity against all bacteria, except S. epidermidis ATCC 12228. The MIC values of the essential oil ranged from 500 μg/mL to 1,000 μg/mL. The findings of our study showed bactericidal activity only against P. vulgaris (MBC=1000 μg/mL) and bacteriostatic activity against P. vulgaris, M. luteus and C. xerosis.
Scanning electron microscopy
The SEM analysis allowed for morphologically identifying structures and to perceive morphological changes of the studied bacteria. There was a change in the structure of the treated bacteria when compared to the negative control. The untreated cells of all microorganisms tested (negative control) showed cell membrane with a regular and smooth surface, whereas the treated ones revealed a severe detrimental effect on the morphology of the cell membrane.
The cells exposed to the essential oil and the reference drugs (neomycin and chloramphenicol) were capable to alter the bacterial cell membrane. The cells exposed to the action of antimicrobial agents have shown deformities in the structure (orifices and appearance of fissures, disintegration of the cell membrane, aggregated cells and cell extravasation) with probable cell destruction (Fig 2).
Fig 2. I: SEM images of P. vulgaris ATCC 13315. II: SEM images of M. luteus ATCC 7468. III: SEM images of C. xerosis IAL105. A: untreated bacterial cells, B: treatment with chloramphenicol, C: treatment with neomycin, D: treatment with essential oil of B. trimera. "a": indicates cleft formation. "b": shows pore formation. "c": shows destroyed cell. "d": shows wrinkled abnormalities. "e": indicates aggregated and deformed cells. "f": indicates disruption and lysis of the membrane integrity. "g": indicates deformed cell. Magnifications: X20,000 (I and II) and X10,000 (III).
DISCUSSION
According to Bakkali et al.26 essential oils are characterized by two or three major components at high concentrations (20 - 70%) compared to other components present in trace amounts. The major compound found in this study was β-pinene (23.4%). It is a bicyclic monoterpene which has been identified as one of the most important bioactive constituents of many essential oils. The antimicrobial effect of β-pinene has been attributed to alterations produced at the membrane level.27 This work has been developed according to the percentage of β-pinene. However, there is some evidence that minor components of essential oils have contributed to the antimicrobial activity, possibly by producing a synergistic or antagonistic effect between other components.28,29
In addition, carquejyl acetate, which accounted for 19.0% of the essential oil, can also make a contribution to the antimicrobial activity. It has been reported that the great commercial value of the essential oil of "carqueja" produced in Brazil has been associated with high carquejyl acetate content.30 Carquejyl acetate and carquejol, which are present in the essential oil, were proposed as a chemotaxonomic marker of this species. Nevertheless, some populations of B. trimera without these compounds have been recently reported, suggesting the existence of a novel chemotype or chemical variations which arise as a result of different environmental conditions.31
Amaral et al.32 found β-pinene, palustrol and carquejyl acetate as the major compounds in the essential oil of B. trimera. Such findings are in agreement with our results which demonstrated β-pinene and carquejyl acetate in higher percentages.
On the other hand, Oliveira et al.11 identified (E) - caryophyllene, germacrene D and bicyclogermacrene as the main compounds. Although those compounds were not the major constituents in the present study, they are present in the chemical composition of the essential oil.
Lago et al.33 found α-humulene (19.44%) as the major constituent and other compounds in lower percentages, such as myrcene (6.09%) and germacrene D (8.86%). Those constituents were found in the present study, but in different concentrations.
Morais and Castanha30 suggested the essential oil of B. trimera is mainly composed by carquejol, carquejyl acetate, α and β-pinene, trans-β-ocimene, nerolidol and spathulenol. Our study revealed the presence of all those compounds, except nerolidol. In contrast, they observed a higher concentration of sesquiterpenes compared to monoterpenes and the presence of ledol (13.7%) in high concentration. Nevertheless, this compound is frequently found in low concentrations in the essential oil of B. trimera, as we found in this study (4.6%).30,34
According to Silva et al.15 the seasonal variations of the essential oil of wild and cultivated B. trimera populations indicated differences in the chemical composition of wild and cultivated flowering samples from a period of March-May showed high percentages of globulol and spathulenol. Wild samples collected from June-February exhibited germacrene D and (E)-caryophyllene as the major constituents, while samples cultivated from June-February contained a high content of ledol.
Furthermore, Silva et al.12 evaluated the effect of different post-harvest processing forms of drugs constituted of parts of "carqueja" on the chemical composition of its essential oil. The concentration of the major constituents of "carqueja" essential oil, spathulenol and ledol, was not affected by the post-harvest treatment. Although they presented different variations, with ledol concentrations decreasing and spathulenol concentrations increasing.
Accordingly, it should be noticed that there are factors which may lead to changes in its chemical composition, such as extraction technique, time of collection, drying of the plant material, soil types, genetic factors and climate.35
Although several researchers reported antimicrobial activity of essential oils or extracts of B. trimera against some bacteria, such as Escherichia coli, Staphylococcus aureus and Streptococcus uberis,36-38 the antimicrobial activity with the establishment of the MIC value of the essential oil against the tested bacteria has not been performed up to now.
Pires34 researched the antimicrobial activity of the essential oil of B. trimera against S. epidermidis ATCC 12228 and M. luteus ATCC 9341. However, this author used the bioautography method as a preliminary biological screening with no quantitative analysis.
Despite the fact that the MIC values of the essential oil were higher than those of chloramphenicol and neomycin, the use of the essential oil of B. trimera may be an alternative as a natural antimicrobial agent in personal care products. The MIC values (500 and 1000 µg/mL) demonstrated to be sufficient inhibiting the growth of bacteria. The use of plant extracts or essential oils by consumers and regulatory agencies is expected and reported to be increasing due to "green consumerism".39 Therefore, as well as some essential oils have pharmacological properties, others can be used as starting compounds which are useful in the chemical, cosmetic and pharmaceutical industries.11
The findings of the SEM suggested that the essential oil of B. trimera could affect the morphology of cells and destroy cell membranes. Such results demonstrated that the essential oil had a destructive effect on P. vulgaris ATCC 13315, M. luteus ATCC 7468 and C. xerosis IAL105 bacterial cells.
Damages to the cell membrane structure and function of the bacteria are probably due to the presence of monoterpenes, which can cause damage to the cell membrane. According to Florão,40 these constituents have a lipophilic character, resulting in the expansion and increase of the fluidity of the membrane and enzymatic inhibition.
In conclusion, the essential oil of Baccharis trimera has a chemical composition complex with a potential application as antimicrobial agent against to bacteria causal of bad body odor in modern human being. Further studies are necessary.
REFERENCES BIBLIOGRAPHIC
1. Biesterbos JWH, Dudzina T, Delmaar CJE, Bakker MI, Russel FGM, Goetz N, et al. Usage patterns of personal care products: Important factors for exposure assessment. Food Chem Toxicol. 2013;55(5):8-17.
2. SPC. Still smelling sweet: deodorant market. England: HPCi Media. 2012.
3. Antignac E, Nohynek GJ, Re T, Clouzeau J, Toutain H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem Toxicol. 2011;49(2):324-41.
4. Manou I, Bouillard L, Devleeschouwer MJ, Barel AO. Evaluation of the preservative properties of Thymus vulgaris essential oil in topically applied formulations under a challenge test. J Appl Microbiol. 1998; 84(3):368-76.
5. Vila R, Santana AI, Pérez-Rosés R, Valderrama A, Castelli MV, Mendonca S, et al. Composition and biological activity of the essential oil from leaves of Plinia cerrocampanensis, a new source of alpha-bisabolol. Bioresour Technol. 2010;101(7):2510-4.
6. Lv F, Liang H, Yuan Q, Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res Int. 2011;44(9):3057-64.
7. Vermaak I, Kamatou GPP, Komane-Mofokeng B, Viljoen AM, Beckett K. African seed oils of commercial importance - Cosmetic applications. S A J Bot. 2011;77(4):920-33.
8. Solórzano-Santos F, Miranda-Novales MG. Essential oils from aromatic herbs as antimicrobial agents. Curr Opin Biotechnol. 2012;23(2):136-41.
9. Aboy AL, Apel MA, Debenedetti S, Francescato L, Rosella MA, Henriques AT. Assay of caffeoylquinic acids in Baccharis trimera by reversed-phase liquid chromatography. J Chromatogr A. 2012; 1219(53):147-53.
10. Biondo TM, Tanae MM, Coletta ED, Lima-Landman MT, Lapa AJ, Souccar C. Antisecretory actions of Baccharis trimera (Less.) DC aqueous extract and isolated compounds: Analysis of underlying mechanisms. J Ethnopharmacol. 2011;136(2):368-73.
11. Oliveira RN, Rehder VLG, Oliveira ASS, Montanari-Júnior I, Carvalho JE, Ruiz ALTG, et al. Schistosoma mansoni: In vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Exp Parasitol. 2012;132(2):135-43.
12. Silva FG, Nascimento VE, Pinto JEBP, Oliveira CBA, Santos MR, Ferri PH. Influência do processamento pós-colheita e armazenamento na composição química da droga vegetal e do óleo essencial de carqueja [Baccharis trimera (Less) D.C.]. Rev Bras Plantas Med. 2010;12(4):436-42.
13. Dias LFT, Melo ES, Hernandes LS, Bacchi EM. Atividades antiúlcera e antioxidante Baccharis trimera (Less) DC (Asteraceae). Rev Bras Farmacogn. 2009;19(1b):309-14.
14. Rodrigues CR, Dias JH, Mello RN, Richter MF, Picada JN, Ferraz AB. Genotoxic and antigenotoxic properties of Baccharis trimera in mice. J Ethnopharmacol. 2009;125(1):97-101.
15. Silva FG, Oliveira CBA, Pinto JEBP, Nascimento VE, Santos SC, Seraphin JC, et al. Seasonal variability in the essential oils of wild and cultivated Baccharis trimera. J Braz Chem Soc. 2007; 18(5):990-7.
16. Schuch LFD, Wiest JM, Garcia EN, Prestes LS, Schramm RC, Coimbra H, et al. Atividade antifúngica de extratos de plantas utilizados por agricultores familiares como antimicrobiano. Acta Sci Vet. 2008;36(3):267-71.
17. Lermen C, Cagnini CZ, Pelisson DC, Girotto D, Rodrigues KPLC, Nicareta L, et al. Ação do óleo essencial obtido da carqueja - Baccharis trimera D.C. (Asteraceae) sobre Cândida albicans. Rev Biol Saúde Unisep. 2009;3(2):30-6.
18. Demo M, Oliva MM, López ML, Zunino MP, Zygadlo JA. Antimicrobial activity of essential oils obtained from aromatic plants of Argentina. Pharm Biol. 2005;43(2):129-34.
19. Cobos MI, Rodriguez JL, Oliva ML, Demo M, Faillaci SM, Zygadlo JA. Composition and antimicrobial activity of the essential oil of Baccharis notosergila. Planta Med. 2001; 67(1):84-6.
20. Albuquerque MRJR, Souza EB, Lins MUDS, Nogueira NAP, Lemos TLG, Silveira ER, et al. Composition and antimicrobial activity of the essential oil from aerial parts of Baccharis trinervis (Lam.) Pers. ARKIVOC. 2004;6:59-65.
21. Hadad M, Zygadlo JA, Lima B, Derita M, Feresin GE, Zacchino SA, et al. Chemical composition and antimicrobial activity of essential oil from Baccharis grisebachii Hieron (Asteraceae). J Chil Chem Soc. 2007;52(2):1186-9.
22. Simões-Pires CA, Debenedetti S, Spegazzini E, Mentz LA, Matzenbacher NI, Limberger RP, et al. Investigation of the essential oil from eight species of Baccharis belonging to sect. Caulopterae (Asteraceae, Astereae): a taxonomic approach. Pl Syst Evol. 2005;253(1):23-32.
23. Adams RP. Identification of essential oil components by Gas Chromatography/Mass Spectrometry. 4th edn. Allured Publ Corp., Carol Stream IL. 2007.
24. Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sökmen A, et al. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol. 2003; 87(2-3):215-20.
25. Gao C, Tian C, Lu Y, Xu J, Luo J, Guo X. Essential oil composition and antimicrobial activity of Sphallerocarpus gracilis seeds against selected food-related bacteria. Food Control. 2011;22(3-4):517-22.
26. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils - A review. Food Chem Toxicol. 2008;46(2):446-75.
27. Belletti N, Kamdem SS, Tabanelli G, Lanciotti R, Gardini F. Modeling of combined effects of citral, linalool and beta-pinene used against Saccharomyces cerevisiae in citrus-based beverages subjected to a mild heat treatment. Int J Food Microbiol. 2010;136(3):283-9.
28. Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 2004;94(3):223-53.
29. Jiang Y, Wu N, Fu YJ, Wang W, Luo M, Zhao CJ, et al. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ Toxicol Pharmacol. 2011;32(1):63-8.
30. Morais LAS, Castanha RF. Composição química do óleo essencial de duas amostras de carqueja (Baccharis sp.) coletadas em Paty do Alferes - Rio de Janeiro. Rev Bras Plantas Med. 2011;13(spe):628-32.
31. Retta D, Gattuso M, Gattuso S, Lira PDL, Baren C, Bandonia A. Volatile constituents of five Baccharis species from Northeastern Argentina. J Braz Chem Soc. 2009;20(7):1379-84.
32. Amaral AS, Mossi AJ, Radünz LL, Treichel H, Teixeira AJ, Lerin LA, et al. Cultivo de carqueja (Baccharis trimera) em solução nutritiva com diferentes concentrações de nitrogênio, fósforo e potássio. Perspectiva. 2010;34(127):25-34.
33. Lago JHG, Romoff P, Fávero OA, Soares MG, Baraldi PT, Corrêa AG, et al. Composição química dos óleos essenciais das folhas de seis espécies do gênero Baccharis de "Campos de Altitude" da Mata Atlântica Paulista. Quim Nova. 2008;31(4):727-30.
34. Pires CAS. Avaliação química e biológica de extratos de Baccharis pertencentes à seção Caulopterae (carquejas). Master's dissertation. Universidade Federal do Rio Grande do Sul, Brazil. 2004.
35. Silva DCMN, Bresciani LFV, Dalagnol RL, Danielski L, Yunes RA, Ferreira SRS. Supercritical fluid extraction of carqueja (Baccharis trimera ) oil: Process parameters and composition profiles. Food Bioprod Process. 2009:87(4):317-26.
36. Duarte MCT, Leme EE, Delarmelina C, Soares AA, Figueira GM, Sartoratto A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J Ethnopharmacol. 2007;111(2):197-201.
37. Martinez MJA, Bessa AL, Benito PB. Biologically active substances from the genus Baccharis L. (Compositae). Stud Nat Prod Chem. 2005;30:703-59.
38. Avancini CAM, Wiest JM, Mundstock E. Atividade bacteriostática e bactericida do decocto de Baccharis trimera (Less.) D.C., Compositae, carqueja, como desinfetante ou anti-séptico. Arq Bras Med Vet Zootec. 2000;52(3):230-4.
39. Arthanari SK, Vanitha J, Ganesh M, Venkateshwaran K, Clercq D. Evaluation of antiviral and cytotoxic activities of methanolic extract of S. grandiflora (Fabaceae) flowers. Asian Pac J Trop Biomed. 2012;2(2):S855-8.
40. Florão A. Avaliação de atividades biológicas de óleos essenciais de quatro espécies de Baccharis, Asteraceae. Master's dissertation. Universidade Federal do Paraná, Brazil. 2006.
Érika Yoko Suzuki: Núcleo de Pesquisa e Inovação em Ciências da Saúde (NUPICS), Faculdade de Farmácia, Universidade Federal de Juiz de Fora, Juiz de Fora-MG, Brazil. Correo electrónico: suzukibarbacena@uol.com.br
Recibido: 14 de
julio de 2015.
Aprobado:
22 de abril de 2016.
Érika Yoko Suzuki: Faculdade de Farmácia, Universidade Federal de Juiz de Fora, Juiz de Fora-MG, Brazil. Correo electrónico: revistamil@infomed.sld.cu